View STRIVE-ON Trial
View more study details through the Clinical Trials website.
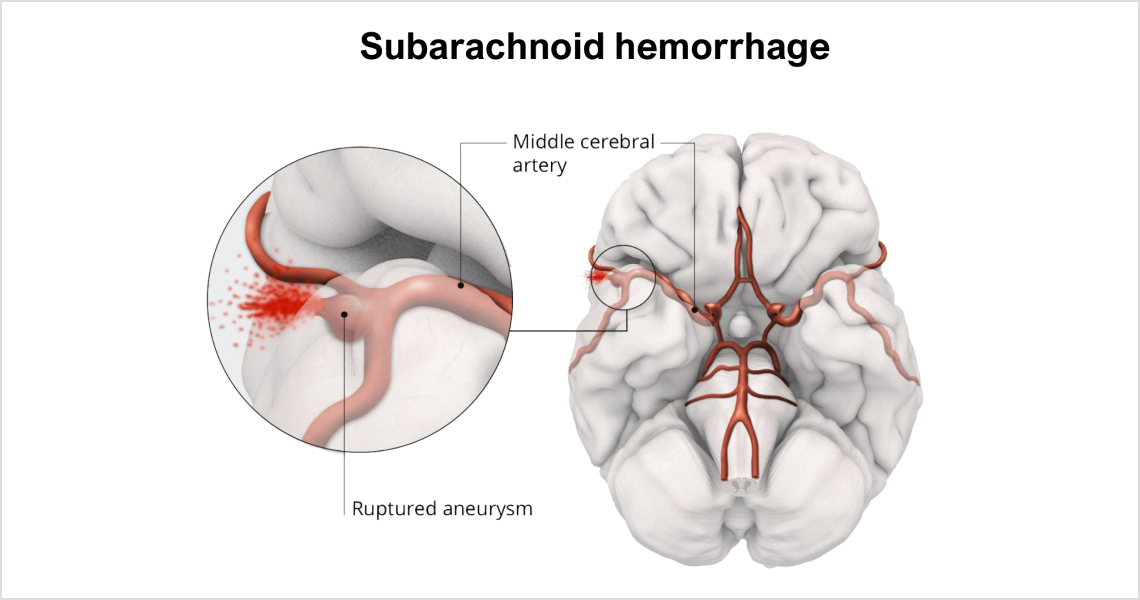
aSAH is bleeding over the surface of the brain in the subarachnoid space between the brain and the skull, which contains blood vessels that supply the brain. A primary cause of such bleeding is the rupture of an aneurysm. Approximately 70% of aSAH patients experience death or dependence, and more than 30% die within one month of hemorrhage. Approximately 50,000 patients in the United States are affected by aSAH per year, based on market research.
STRIVE-ON (NCT05995405) will study a parallel group of 100 patients hospitalized aSAH in a randomized (1:1 ratio) open-label comparison between oral nimodipine and intravenous (IV) GTx-104.

View more study details through the Clinical Trials website.