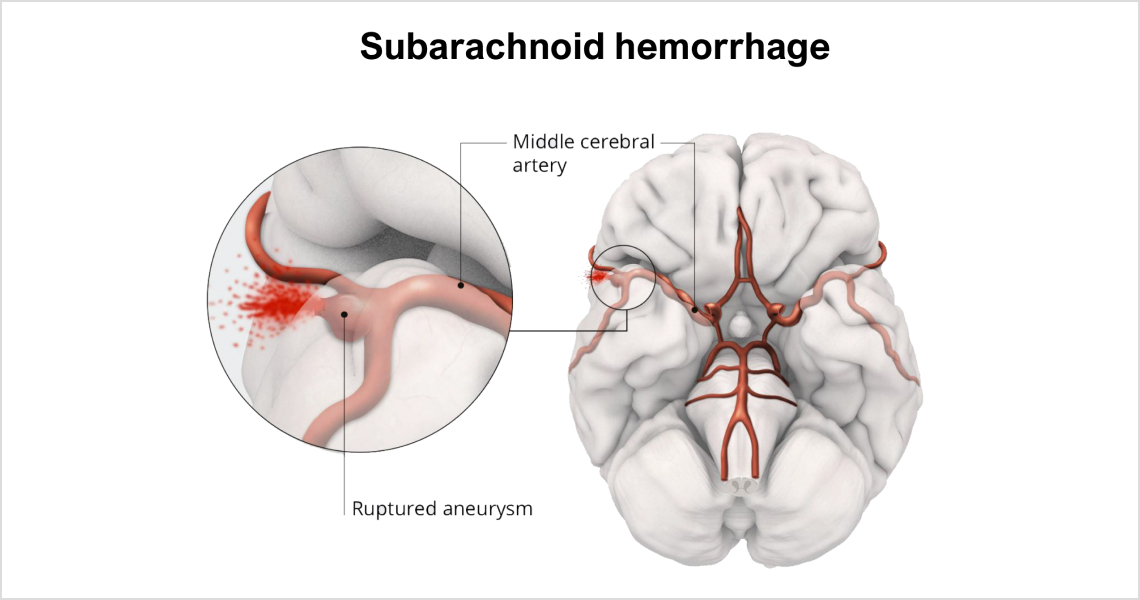
aSAH occurs suddenly and is caused by a ruptured aneurysm that bleeds in the subarachnoid space between the brain and skull.
Immediate surgical and pharmacotherapy intervention is key as the condition deteriorates quickly.
Currently, the only SoC is oral nimodipine therapy indicated for up to 21 days.