EXHIBIT 99.1
Published on March 3, 2025
Exhibit 99.1

Corporate Presentation March 2025

Summary 2 Forward Looking Statements Statements in this presentation that are
not statements of historical or current fact constitute "forward-looking statements" within the meaning of the U.S. Private Securities Litigation Reform Act of 1995, as amended, Section 27A of the Securities Act of 1933, as amended, and Section
21E of the Securities Exchange Act of 1934, as amended, and "forward-looking information" within the meaning of Canadian securities laws (collectively, "forward-looking statements"). Such forward looking statements involve known and unknown
risks, uncertainties, and other factors that could cause the actual results of Grace Therapeutics, Inc. to be materially different from historical results or from any future results expressed or implied by such forward-looking statements. In
addition to statements which explicitly describe such risks and uncertainties, readers are urged to consider statements containing the terms "believes," "belief," "expects," "intends," "anticipates," "estimates", "potential," "should," "may,"
"will," "plans," "continue", "targeted" or other similar expressions to be uncertain and forward-looking. Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this
presentation. The forward-looking statements in this presentation, including, but not limited to, statements regarding the Company’s expected cash runway, preliminary estimates of the Company’s cash and cash equivalents, the future prospects of
the Company’s GTx-104 drug candidate, the timing of the Company's anticipated NDA submission for GTx-104 with the FDA, GTx-104's potential to bring enhanced treatment options to patients suffering from aneurysmal subarachnoid hemorrhage
(“aSAH”), GTx-104’s potential to be administered to improve the management of hypotension in patients with aSAH, the ability of GTx-104 to achieve a pharmacokinetic and safety profile similar to the oral form of nimodipine, GTx-104’s potential
to provide improved bioavailability and the potential for reduced use of rescue therapies, GTx-104’s potential to achieve pharmacoeconomic benefit over the oral form of nimodipine, GTx-104’s commercial prospects, the Company’s pre-commercial
launch strategy for GTx104, the future prospects of the Company’s GTx-102 drug candidate, GTx-102’s potential to provide clinical benefits to decrease symptoms associated with Ataxia Telangiectasia, GTx-102’s potential ease of drug
administration, the timing and outcomes of a Phase 3 efficacy and safety study for GTx-102, the timing of an NDA filing for GTx-102, the size of the addressable market for GTx-104 and GTx 102, and any future patent and other intellectual
property filings made by the Company for new developments are based upon Grace Therapeutics, Inc.’s current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results and the timing of events
could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, including, without limitation: (i) the success and timing of regulatory submissions of the STRIVE-ON Phase 3
safety trial for GTx-104; (ii) regulatory requirements or developments and the outcome and timing of the proposed NDA application for GTx-104; (iii) changes to clinical trial designs and regulatory pathways; (iv) legislative, regulatory,
political and economic developments; and, (v) actual costs associated with Grace Therapeutics clinical trials as compared to management's current expectations. The foregoing list of important factors that could cause actual events to differ
from expectations should not be construed as exhaustive and should be read in conjunction with statements that are included herein and elsewhere, including the risk factors detailed in the "Special Note Regarding Forward-Looking Statements,"
"Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operations" sections of the Company's Annual Report on Form 10-K for the fiscal year ended March 31, 2024, Quarterly Report on Form 10-Q for the
quarterly period ended June 30, 2024, the Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2024, the Quarterly Reports on Form 10-Q and Form 10-Q/A for the quarterly period ended December 31, 2024 and other documents
that have been and will be filed by Grace Therapeutics, Inc. from time to time with the Securities and Exchange Commission and Canadian securities regulators. All forward-looking statements contained in this presentation speak only as of the
date on which they were made. Grace Therapeutics undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made, except as required by applicable securities
laws.
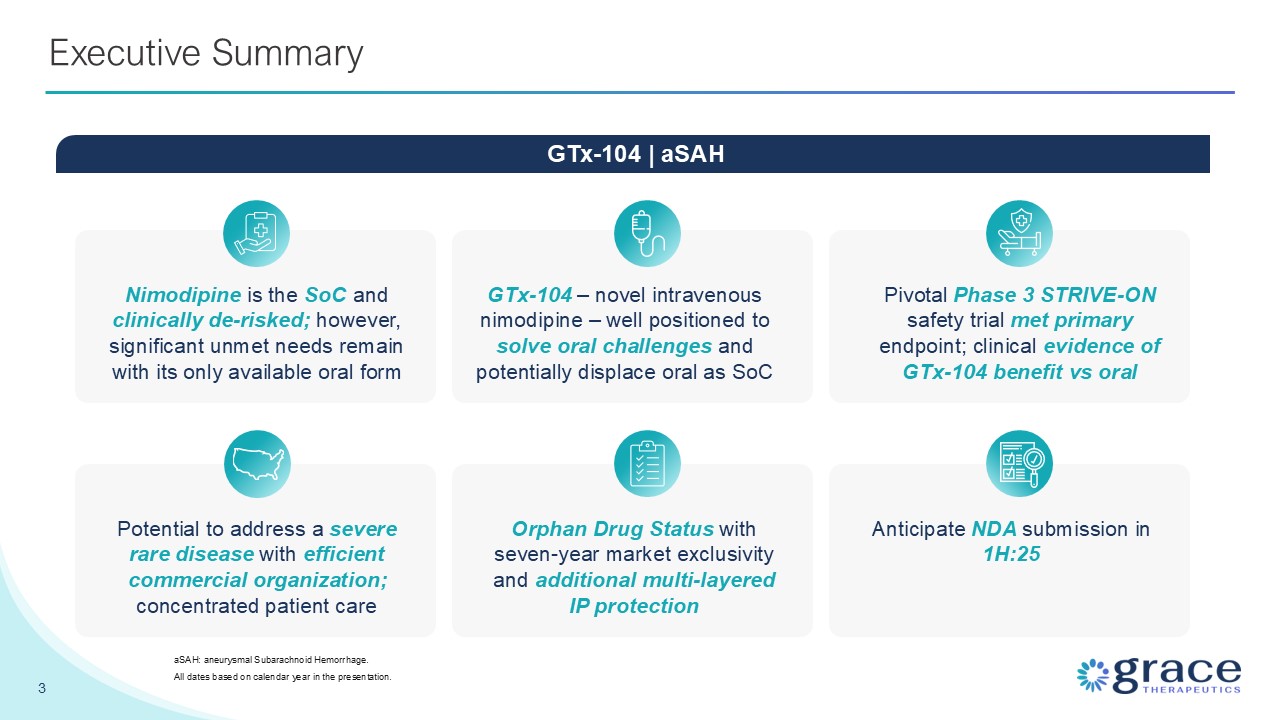
Summary GTx-104 – novel intravenous nimodipine – well positioned to solve oral
challenges and potentially displace oral as SoC Nimodipine is the SoC and clinically de-risked; however, significant unmet needs remain with its only available oral form Pivotal Phase 3 STRIVE-ON safety trial met primary endpoint; clinical
evidence of GTx-104 benefit vs oral 3 Executive Summary aSAH: aneurysmal Subarachnoid Hemorrhage. All dates based on calendar year in the presentation. GTx-104 | aSAH Potential to address a severe rare disease with efficient commercial
organization; concentrated patient care Orphan Drug Status with seven-year market exclusivity and additional multi-layered IP protection Anticipate NDA submission in 1H:25

Experienced Leadership Team 4 Carrie D'Andrea VP Clinical Operations Loch
Macdonald, MD, PhD Chief Medical Officer Prashant Kohli Chief Executive Officer Amresh Kumar, PhD VP Program Management Robert J. DelAversano Principal Financial Officer and Principal Accounting Officer Alejandro A. Rabinstein, MD Alex
Choi, MD Andrew Ducruet, MD Sherry H-Y Chou, MD W. Taylor Kimberly, MD, PhD Management Team Scientific Advisory Board Deep aSAH Expertise in Research, Commercial, Drug & AHA Care Guidelines Development
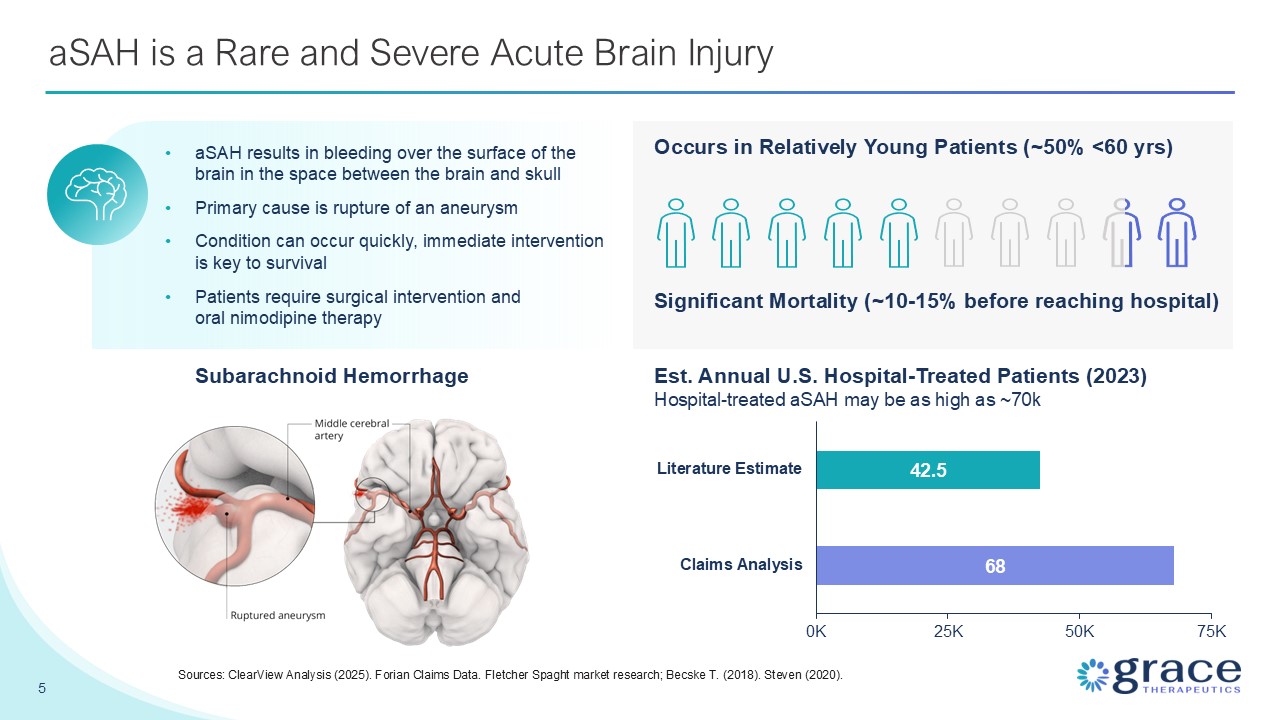
aSAH is a Rare and Severe Acute Brain Injury Subarachnoid Hemorrhage aSAH
results in bleeding over the surface of the brain in the space between the brain and skull Primary cause is rupture of an aneurysm Condition can occur quickly, immediate intervention is key to survival Patients require surgical intervention
and oral nimodipine therapy 5 Sources: ClearView Analysis (2025). Forian Claims Data. Fletcher Spaght market research; Becske T. (2018). Steven (2020). Occurs in Relatively Young Patients (~50% <60 yrs) Significant Mortality (~10-15%
before reaching hospital) Est. Annual U.S. Hospital-Treated Patients (2023) Hospital-treated aSAH may be as high as ~70k

Oral Nimodipine – The aSAH Standard of Care for >3 Decades 6 Sources: Hoh
(2023). Hernandez-Duran (2019). Sandow (2016). DCI: Delayed Cerebral Infarction The Joint Commision is a hospital accredation agency 2023 AHA/ASA GuidelinesFor the management of patients with aSAH Nimodipine is the only approved therapy to
improve neurological outcomes Limited use of off-label therapies due to The Joint Commission monitoring adherence to care guidelines
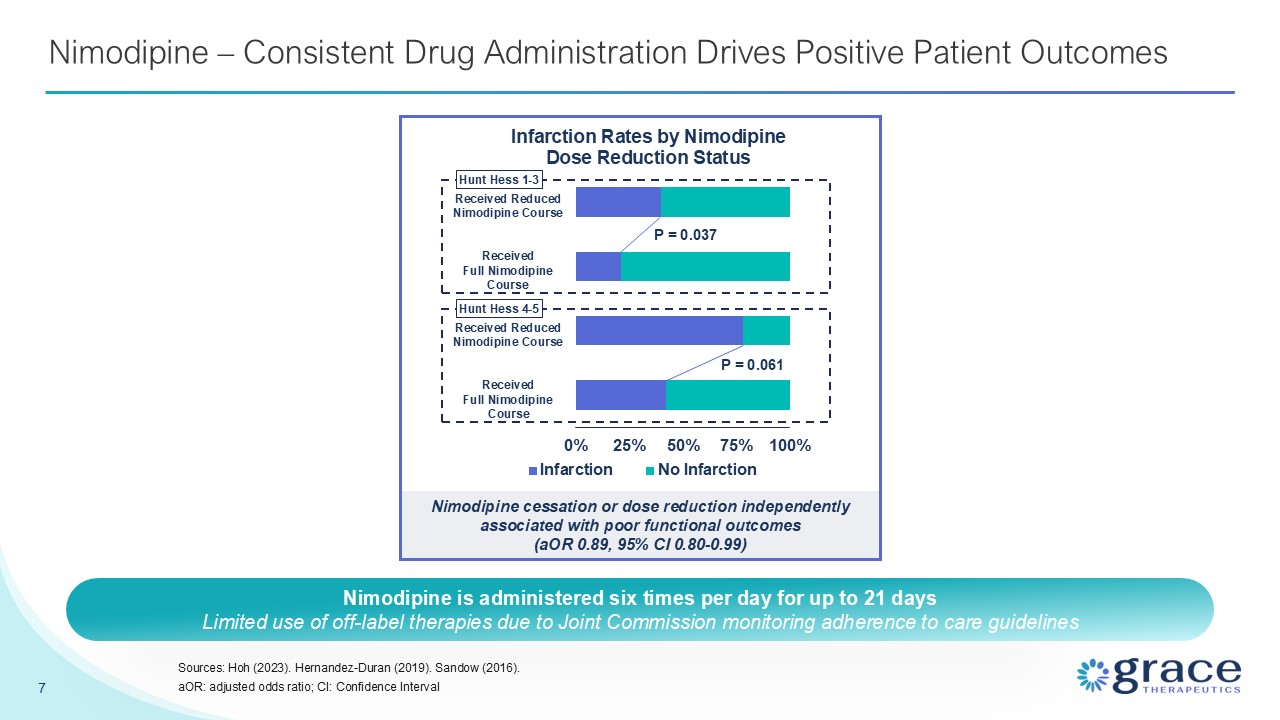
Nimodipine – Consistent Drug Administration Drives Positive Patient
Outcomes 7 Sources: Hoh (2023). Hernandez-Duran (2019). Sandow (2016). aOR: adjusted odds ratio; CI: Confidence Interval Nimodipine cessation or dose reduction independently associated with poor functional outcomes (aOR 0.89, 95% CI
0.80-0.99) P = 0.037 P = 0.061 Received Reduced Nimodipine Course Received Full Nimodipine Course Received Reduced Nimodipine Course Received Full Nimodipine Course Hunt Hess 1-3 Hunt Hess 4-5 Nimodipine is administered six times per
day for up to 21 days Limited use of off-label therapies due to Joint Commission monitoring adherence to care guidelines
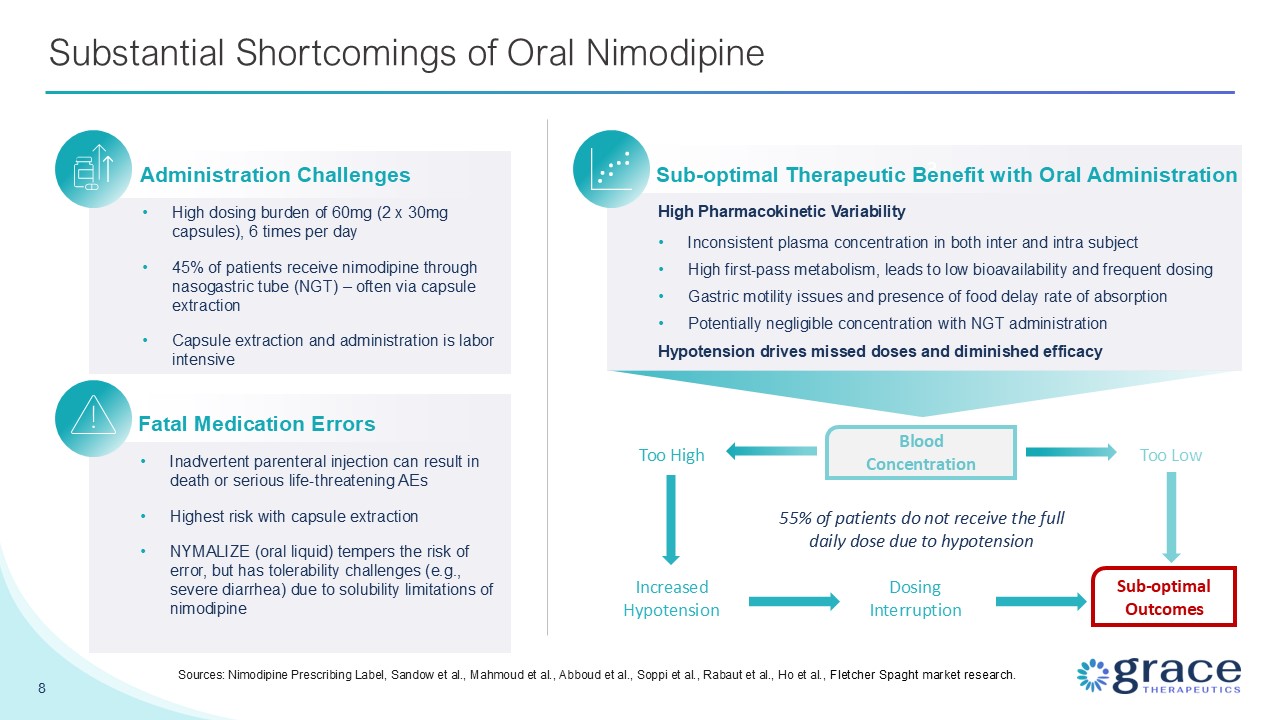
8 Substantial Shortcomings of Oral Nimodipine Sources: Nimodipine Prescribing
Label, Sandow et al., Mahmoud et al., Abboud et al., Soppi et al., Rabaut et al., Ho et al., Fletcher Spaght market research. Administration Challenges High dosing burden of 60mg (2 x 30mg capsules), 6 times per day 45% of patients receive
nimodipine through nasogastric tube (NGT) – often via capsule extraction Capsule extraction and administration is labor intensive Dosing Interruption Increased Hypotension Too High Fatal Medication Errors Inadvertent parenteral injection
can result in death or serious life-threatening AEs Highest risk with capsule extraction NYMALIZE (oral liquid) tempers the risk of error, but has tolerability challenges (e.g., severe diarrhea) due to solubility limitations of
nimodipine 3 Sub-optimal Therapeutic Benefit with Oral Administration High Pharmacokinetic Variability Inconsistent plasma concentration in both inter and intra subject High first-pass metabolism, leads to low bioavailability and frequent
dosing Gastric motility issues and presence of food delay rate of absorption Potentially negligible concentration with NGT administration Hypotension drives missed doses and diminished efficacy Blood Concentration 55% of patients do not
receive the full daily dose due to hypotension Sub-optimal Outcomes Too Low
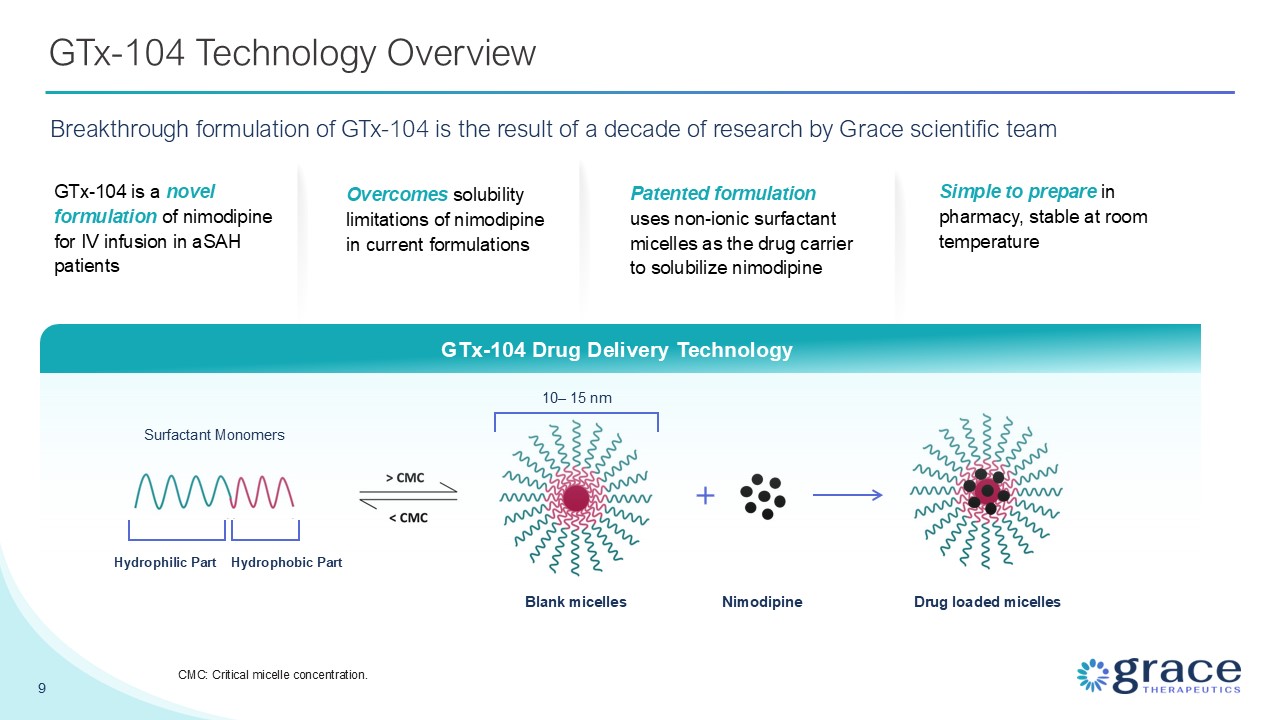
GTx-104 Technology Overview Breakthrough formulation of GTx-104 is the result of
a decade of research by Grace scientific team GTx-104 Drug Delivery Technology Drug loaded micelles Nimodipine 10– 15 nm Blank micelles Surfactant Monomers Hydrophilic Part Hydrophobic Part GTx-104 is a novel formulation of nimodipine
for IV infusion in aSAH patients Overcomes solubility limitations of nimodipine in current formulations Patented formulation uses non-ionic surfactant micelles as the drug carrier to solubilize nimodipine Simple to prepare in pharmacy,
stable at room temperature 9 CMC: Critical micelle concentration.
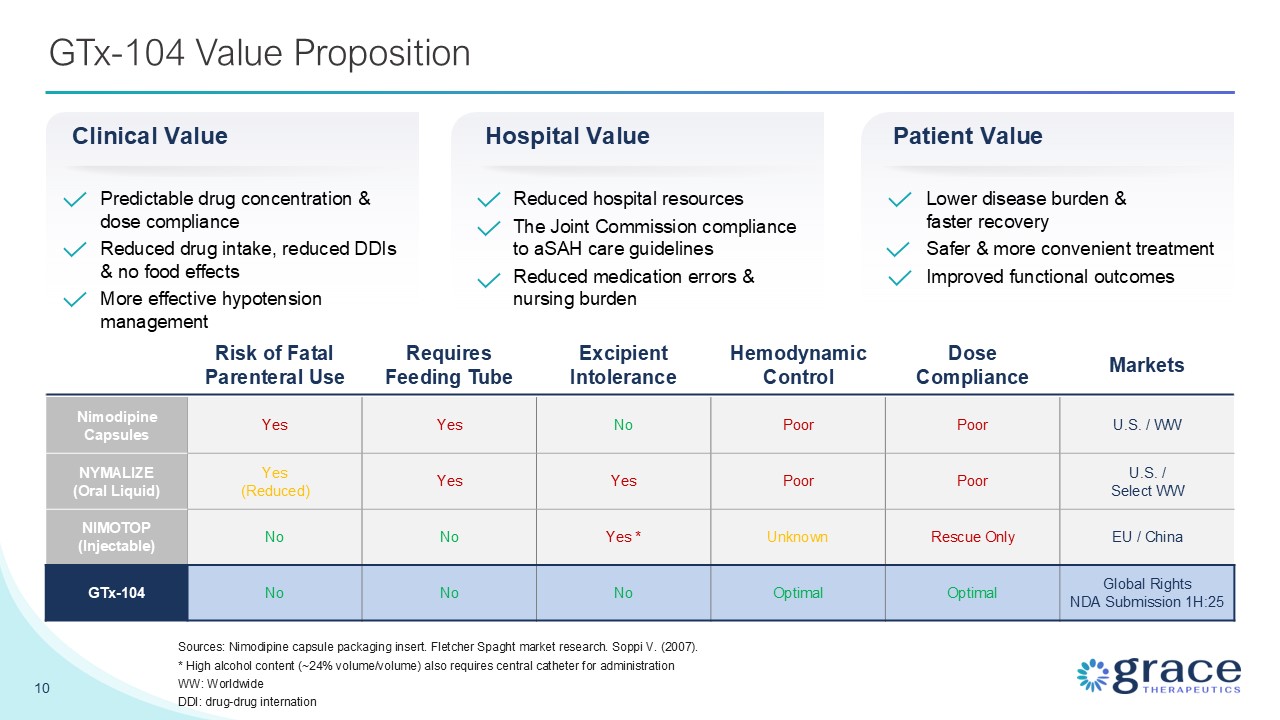
GTx-104 Value Proposition 10 Risk of Fatal Parenteral Use Requires Feeding
Tube Excipient Intolerance Hemodynamic Control Dose Compliance Markets Nimodipine Capsules Yes Yes No Poor Poor U.S. / WW NYMALIZE (Oral Liquid) Yes (Reduced) Yes Yes Poor Poor U.S. / Select WW NIMOTOP
(Injectable) No No Yes * Unknown Rescue Only EU / China GTx-104 No No No Optimal Optimal Global Rights NDA Submission 1H:25 Sources: Nimodipine capsule packaging insert. Fletcher Spaght market research. Soppi V. (2007). * High
alcohol content (~24% volume/volume) also requires central catheter for administration WW: Worldwide DDI: drug-drug internation Predictable drug concentration & dose compliance Reduced drug intake, reduced DDIs & no food
effects More effective hypotension management Clinical Value Hospital Value Reduced hospital resources The Joint Commission compliance to aSAH care guidelines Reduced medication errors & nursing burden Patient Value Lower disease
burden & faster recovery Safer & more convenient treatment Improved functional outcomes
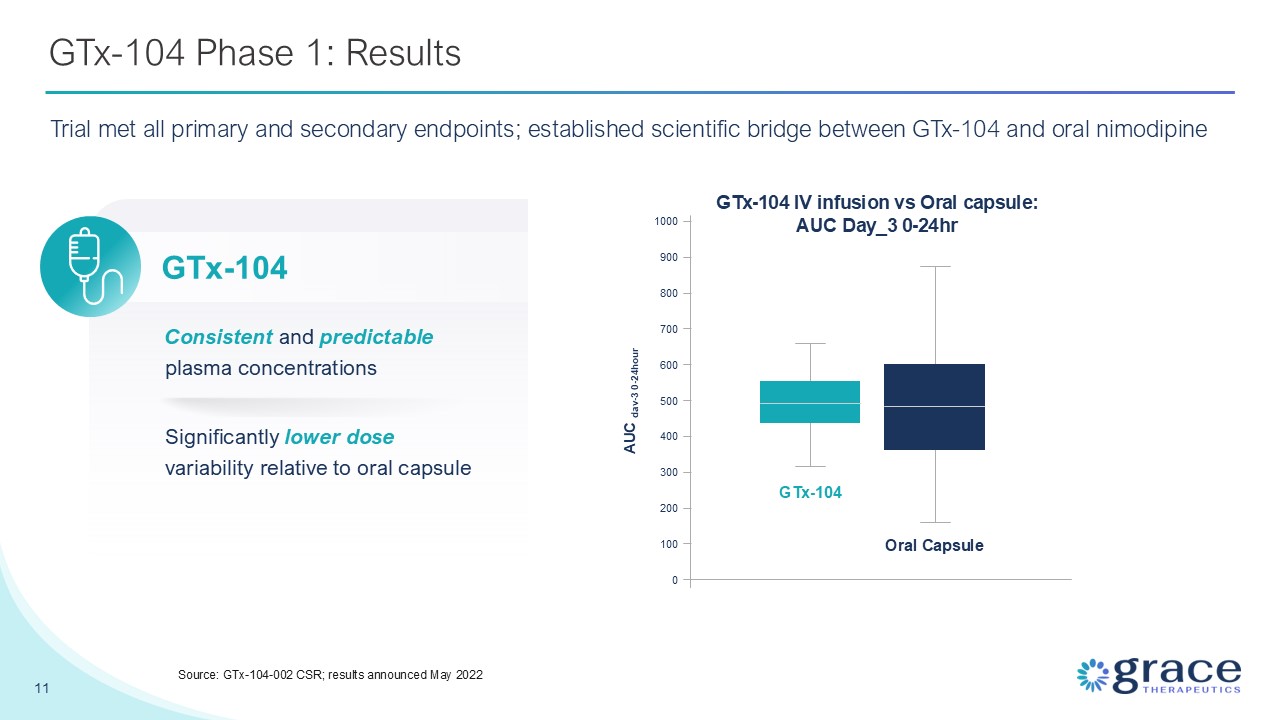
GTx-104 Phase 1: Results 11 Source: GTx-104-002 CSR; results announced May
2022 Significantly lower dose variability relative to oral capsule Consistent and predictable plasma concentrations GTx-104 IV infusion vs Oral capsule: AUC Day_3 0-24hr GTx-104 Oral
Capsule 0 100 200 300 400 500 600 700 800 900 1000 AUC dav-3 0-24hour GTx-104 Trial met all primary and secondary endpoints; established scientific bridge between GTx-104 and oral nimodipine

STRIVE-ON Phase 3 Trial
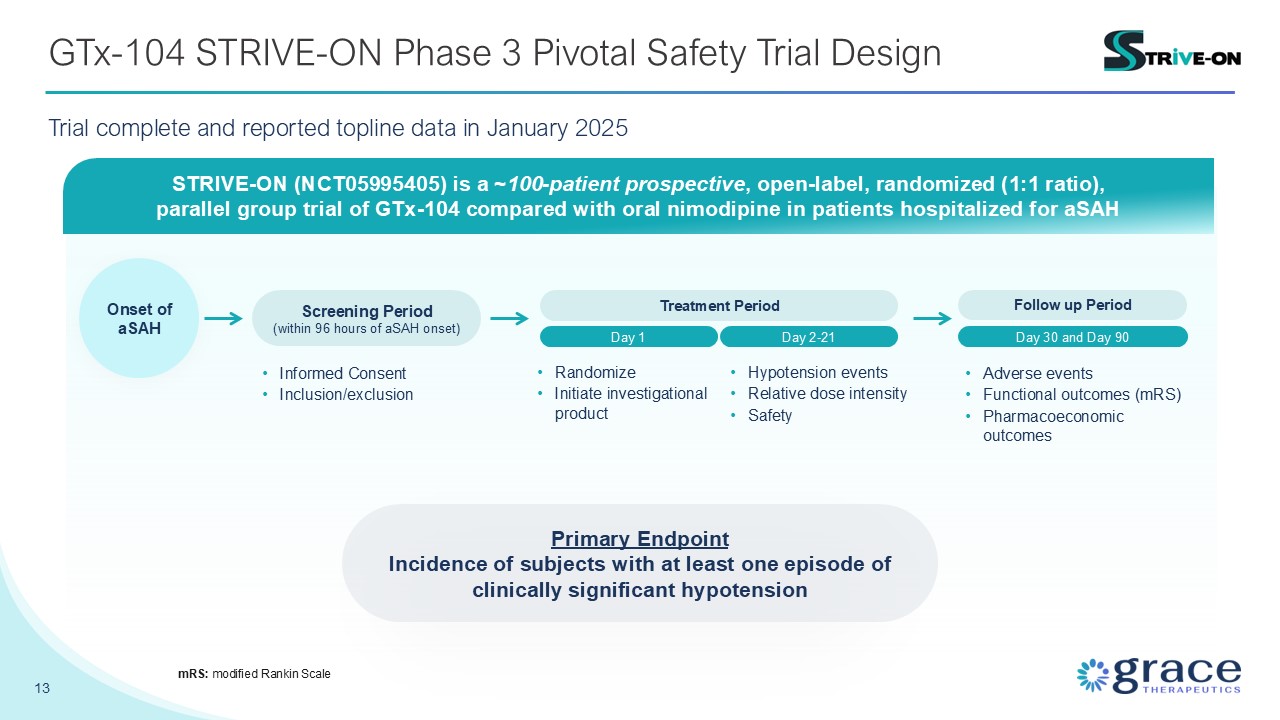
GTx-104 STRIVE-ON Phase 3 Pivotal Safety Trial Design 13 mRS: modified Rankin
Scale STRIVE-ON (NCT05995405) is a ~100-patient prospective, open-label, randomized (1:1 ratio), parallel group trial of GTx-104 compared with oral nimodipine in patients hospitalized for aSAH Screening Period (within 96 hours of aSAH
onset) Day 1 Treatment Period Day 2-21 Onset of aSAH Follow up Period Day 30 and Day 90 Primary Endpoint Incidence of subjects with at least one episode of clinically significant hypotension Informed
Consent Inclusion/exclusion Randomize Initiate investigational product Hypotension events Relative dose intensity Safety Adverse events Functional outcomes (mRS) Pharmacoeconomic outcomes Trial complete and reported topline data in
January 2025
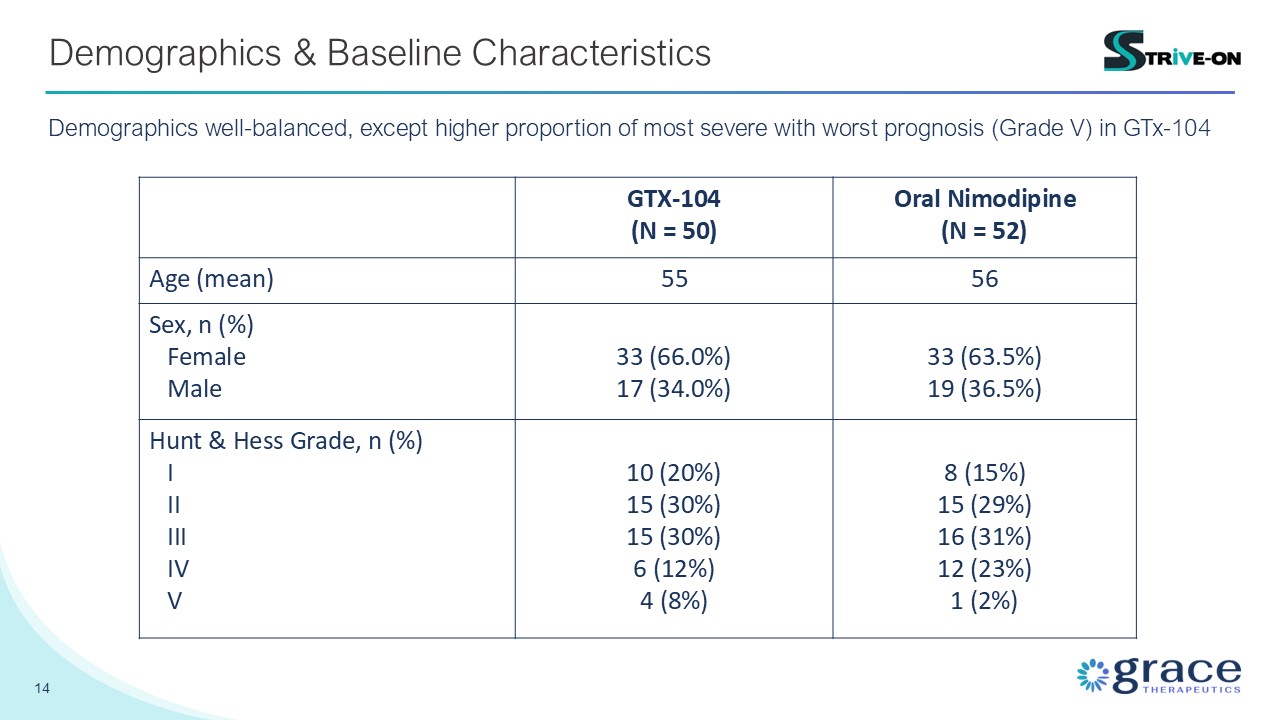
14 Demographics & Baseline Characteristics GTX-104 (N = 50) Oral
Nimodipine (N = 52) Age (mean) 55 56 Sex, n (%) Female Male 33 (66.0%) 17 (34.0%) 33 (63.5%) 19 (36.5%) Hunt & Hess Grade, n (%) I II III IV V 10 (20%) 15 (30%) 15 (30%) 6 (12%) 4 (8%) 8 (15%) 15 (29%) 16
(31%) 12 (23%) 1 (2%) Demographics well-balanced, except higher proportion of most severe with worst prognosis (Grade V) in GTx-104
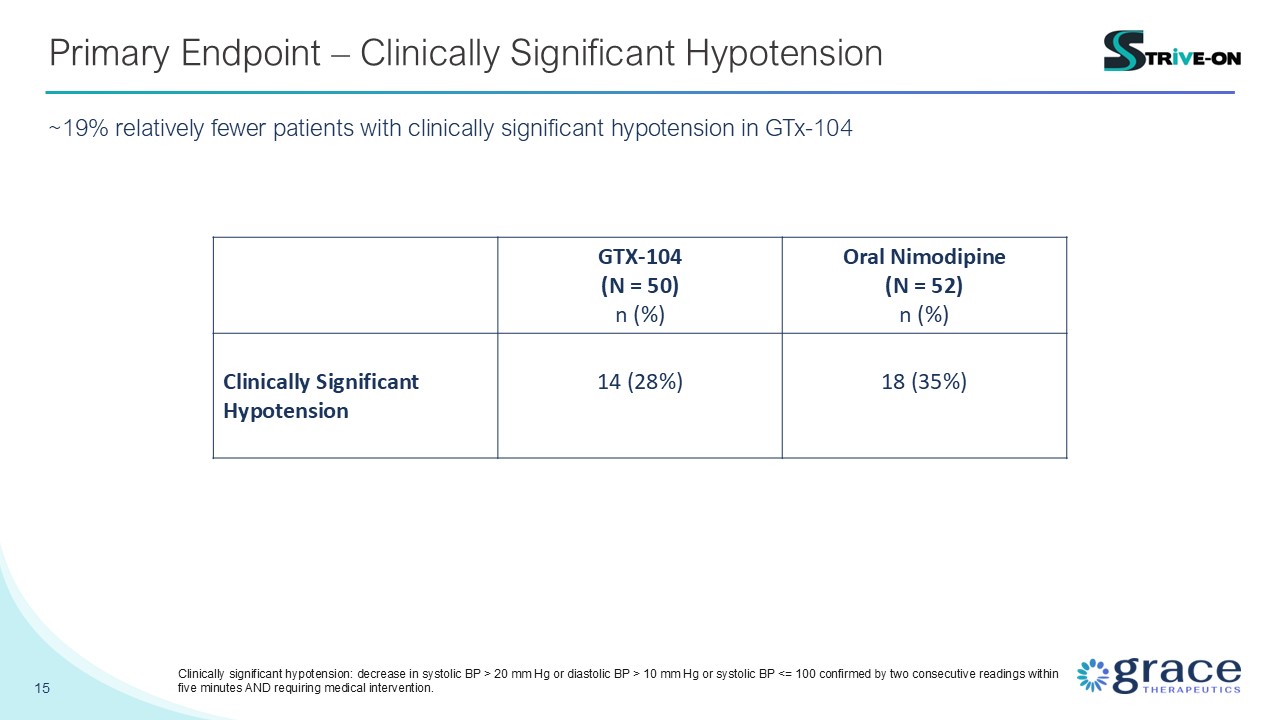
15 Primary Endpoint – Clinically Significant Hypotension ~19% relatively fewer
patients with clinically significant hypotension in GTx-104 GTX-104 (N = 50) n (%) Oral Nimodipine (N = 52) n (%) Clinically Significant Hypotension 14 (28%) 18 (35%) Clinically significant hypotension: decrease in systolic BP > 20
mm Hg or diastolic BP > 10 mm Hg or systolic BP <= 100 confirmed by two consecutive readings within five minutes AND requiring medical intervention.
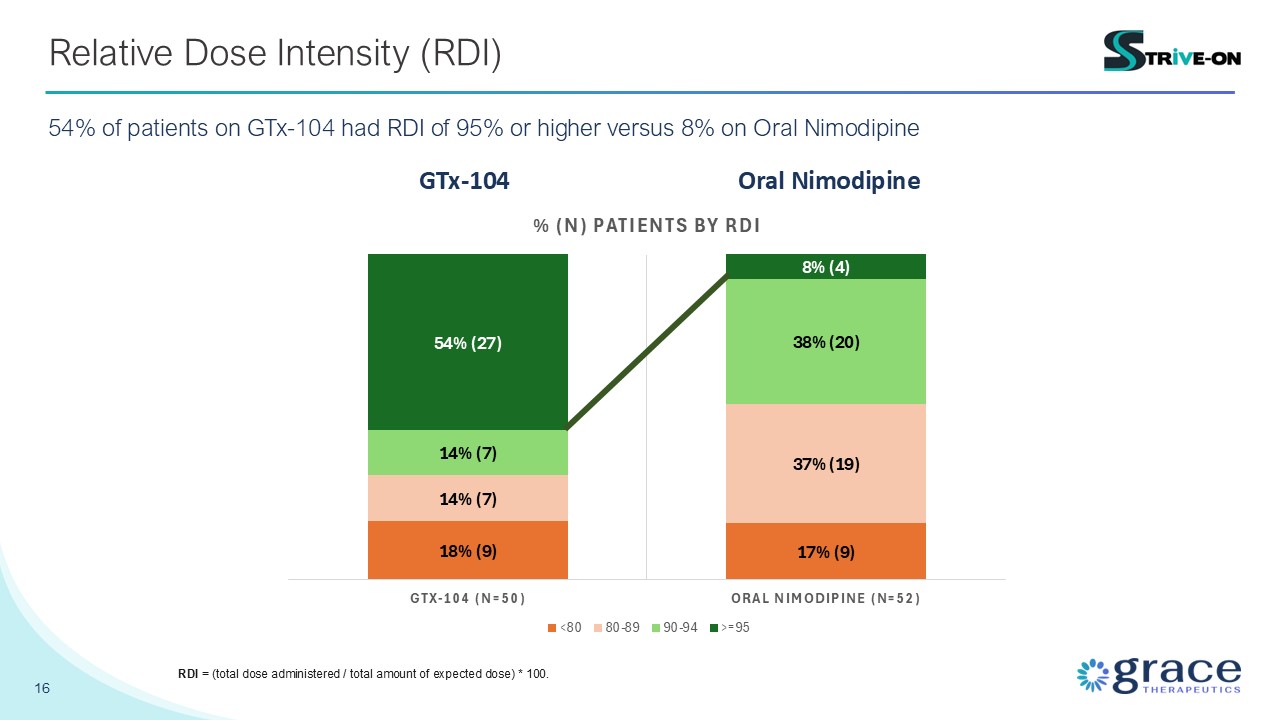
16 Relative Dose Intensity (RDI) 54% of patients on GTx-104 had RDI of 95% or
higher versus 8% on Oral Nimodipine GTx-104 Oral Nimodipine RDI = (total dose administered / total amount of expected dose) * 100.

17 Clinical Outcomes – mRS (day 90) ~29% relative increase in patients with good
recovery in GTx-104 ~29% * 3 patients did not complete physician-conducted mRS at day-90. However, all 3 were confirmed alive at day-90 ** 6 patients did not complete physician-conducted mRS at day-90. 5 were confirmed alive at day-90, and 1
survival status was unknown
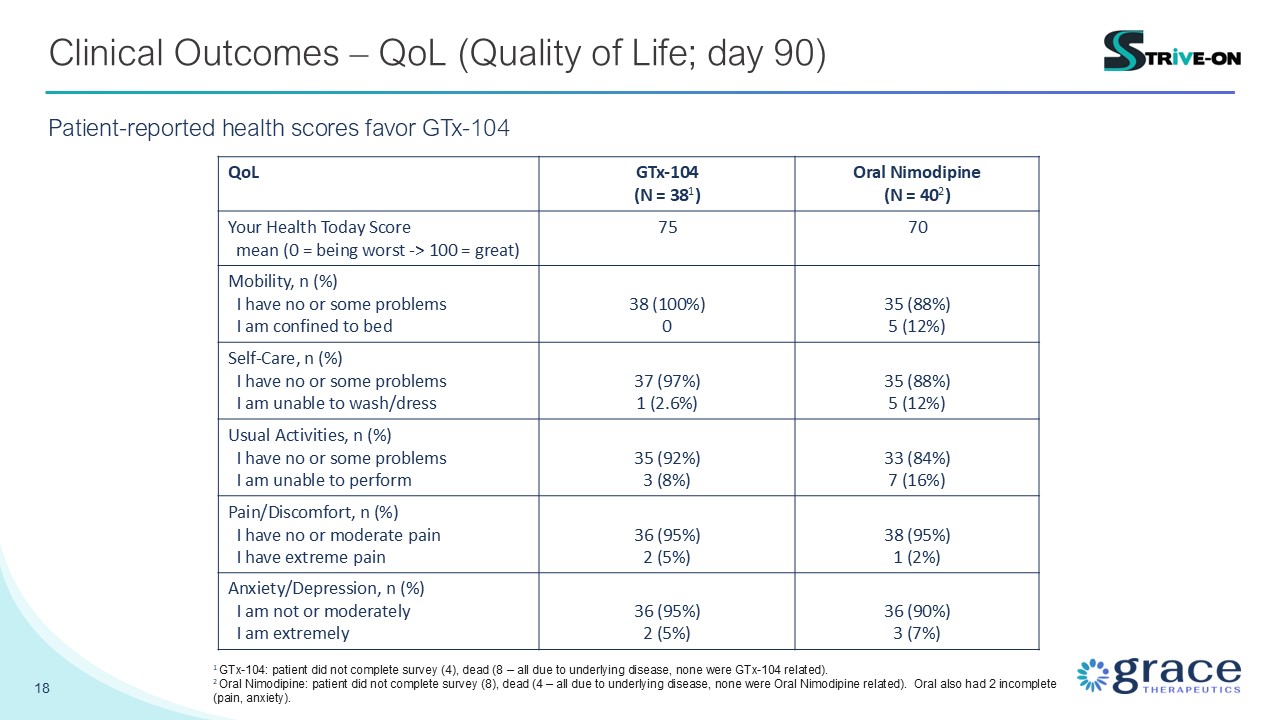
18 Clinical Outcomes – QoL (Quality of Life; day 90) Patient-reported health
scores favor GTx-104 QoL GTx-104 (N = 381) Oral Nimodipine (N = 402) Your Health Today Score mean (0 = being worst -> 100 = great) 75 70 Mobility, n (%) I have no or some problems I am confined to bed 38 (100%) 0 35
(88%) 5 (12%) Self-Care, n (%) I have no or some problems I am unable to wash/dress 37 (97%) 1 (2.6%) 35 (88%) 5 (12%) Usual Activities, n (%) I have no or some problems I am unable to perform 35 (92%) 3 (8%) 33 (84%) 7
(16%) Pain/Discomfort, n (%) I have no or moderate pain I have extreme pain 36 (95%) 2 (5%) 38 (95%) 1 (2%) Anxiety/Depression, n (%) I am not or moderately I am extremely 36 (95%) 2 (5%) 36 (90%) 3 (7%) 1 GTx-104: patient
did not complete survey (4), dead (8 – all due to underlying disease, none were GTx-104 related). 2 Oral Nimodipine: patient did not complete survey (8), dead (4 – all due to underlying disease, none were Oral Nimodipine related). Oral also
had 2 incomplete (pain, anxiety).
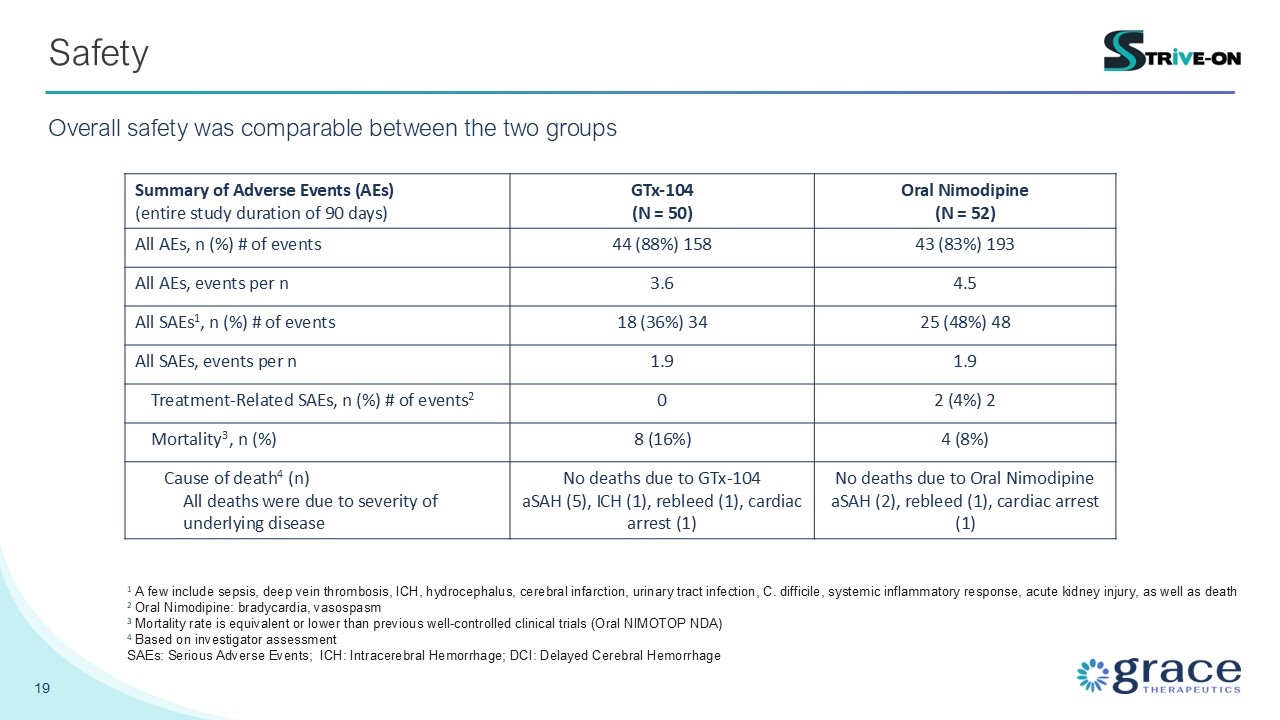
19 Safety Overall safety was comparable between the two groups Summary of
Adverse Events (AEs) (entire study duration of 90 days) GTx-104 (N = 50) Oral Nimodipine (N = 52) All AEs, n (%) # of events 44 (88%) 158 43 (83%) 193 All AEs, events per n 3.6 4.5 All SAEs1, n (%) # of events 18 (36%) 34 25 (48%)
48 All SAEs, events per n 1.9 1.9 Treatment-Related SAEs, n (%) # of events2 0 2 (4%) 2 Mortality3, n (%) 8 (16%) 4 (8%) Cause of death4 (n) All deaths were due to severity of underlying disease No deaths due to GTx-104 aSAH
(5), ICH (1), rebleed (1), cardiac arrest (1) No deaths due to Oral Nimodipine aSAH (2), rebleed (1), cardiac arrest (1) 1 A few include sepsis, deep vein thrombosis, ICH, hydrocephalus, cerebral infarction, urinary tract infection, C.
difficile, systemic inflammatory response, acute kidney injury, as well as death 2 Oral Nimodipine: bradycardia, vasospasm 3 Mortality rate is equivalent or lower than previous well-controlled clinical trials (Oral NIMOTOP NDA) 4 Based on
investigator assessment SAEs: Serious Adverse Events; ICH: Intracerebral Hemorrhage; DCI: Delayed Cerebral Hemorrhage
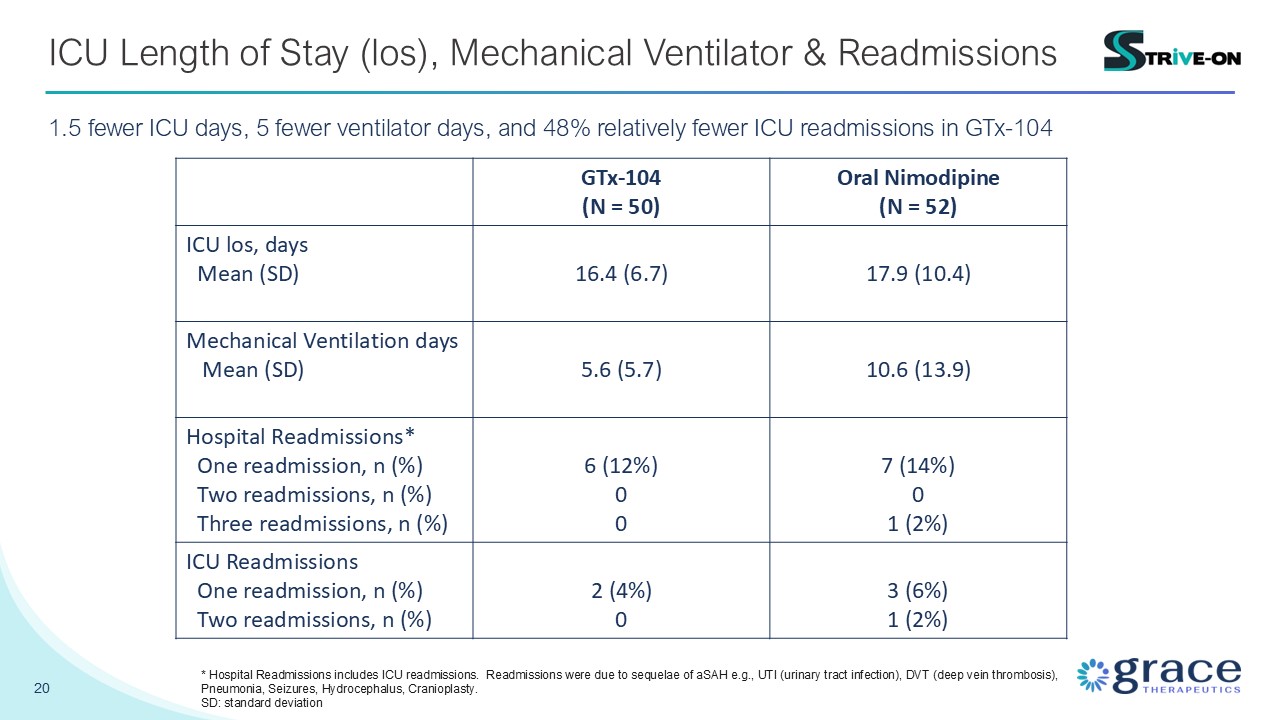
20 ICU Length of Stay (los), Mechanical Ventilator & Readmissions 1.5 fewer
ICU days, 5 fewer ventilator days, and 48% relatively fewer ICU readmissions in GTx-104 GTx-104 (N = 50) Oral Nimodipine (N = 52) ICU los, days Mean (SD) 16.4 (6.7) 17.9 (10.4) Mechanical Ventilation days Mean (SD) 5.6
(5.7) 10.6 (13.9) Hospital Readmissions* One readmission, n (%) Two readmissions, n (%) Three readmissions, n (%) 6 (12%) 0 0 7 (14%) 0 1 (2%) ICU Readmissions One readmission, n (%) Two readmissions, n (%) 2 (4%) 0 3
(6%) 1 (2%) * Hospital Readmissions includes ICU readmissions. Readmissions were due to sequelae of aSAH e.g., UTI (urinary tract infection), DVT (deep vein thrombosis), Pneumonia, Seizures, Hydrocephalus, Cranioplasty. SD: standard
deviation
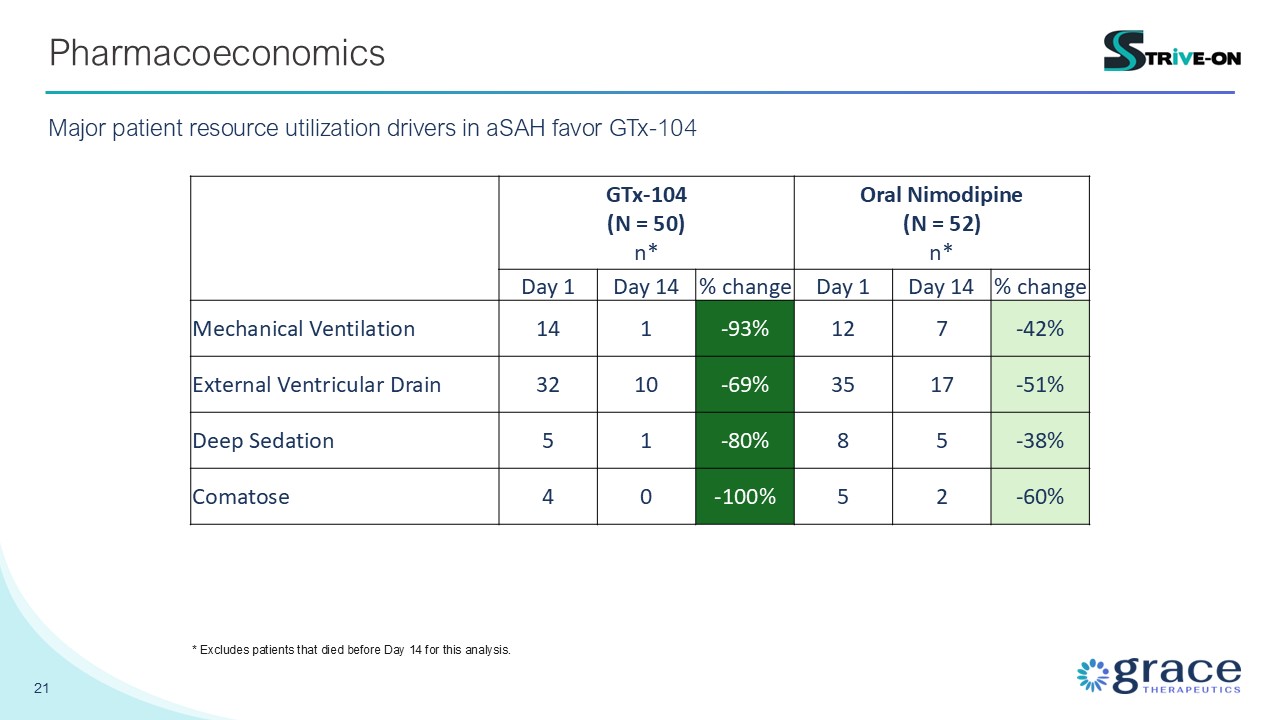
21 Pharmacoeconomics Major patient resource utilization drivers in aSAH favor
GTx-104 GTx-104 (N = 50) n* Oral Nimodipine (N = 52) n* Day 1 Day 14 % change Day 1 Day 14 % change Mechanical Ventilation 14 1 -93% 12 7 -42% External Ventricular Drain 32 10 -69% 35 17 -51% Deep
Sedation 5 1 -80% 8 5 -38% Comatose 4 0 -100% 5 2 -60% * Excludes patients that died before Day 14 for this analysis.

Commercial Preparation
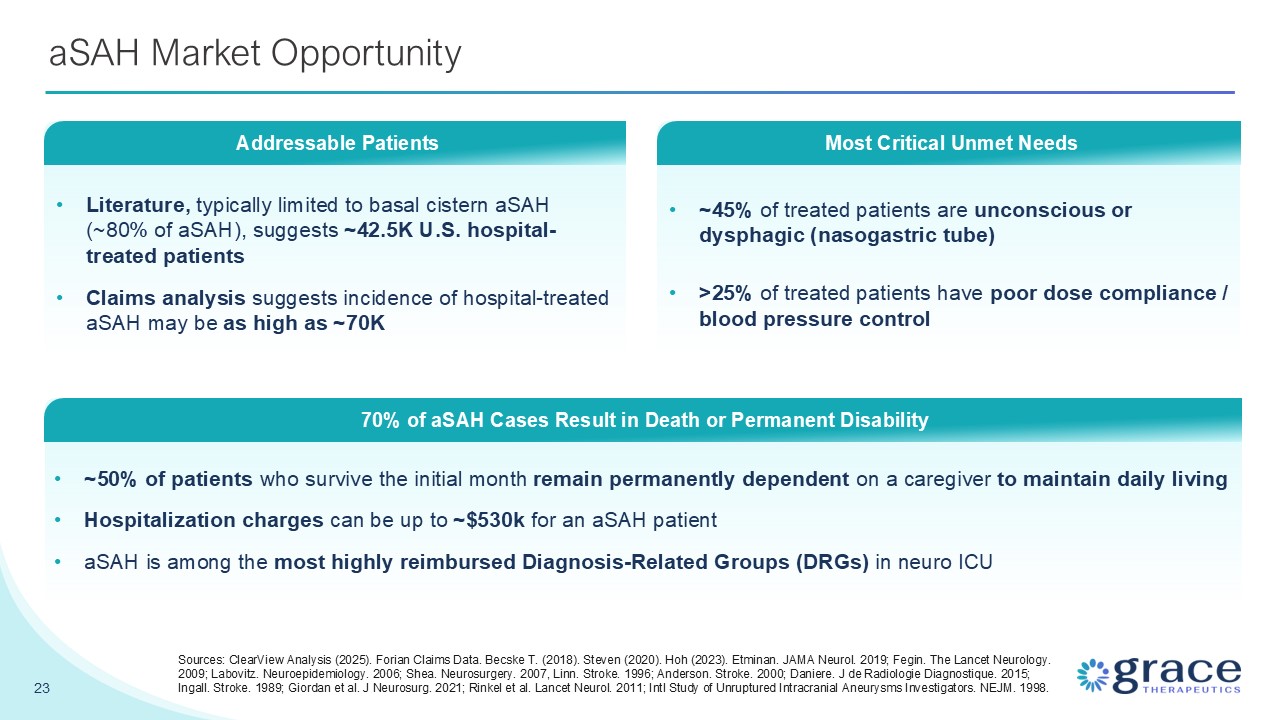
~45% of treated patients are unconscious or dysphagic (nasogastric tube) >25%
of treated patients have poor dose compliance / blood pressure control aSAH Market Opportunity 23 Literature, typically limited to basal cistern aSAH (~80% of aSAH), suggests ~42.5K U.S. hospital-treated patients Claims analysis suggests
incidence of hospital-treated aSAH may be as high as ~70K Addressable Patients ~50% of patients who survive the initial month remain permanently dependent on a caregiver to maintain daily living Hospitalization charges can be up to ~$530k
for an aSAH patient aSAH is among the most highly reimbursed Diagnosis-Related Groups (DRGs) in neuro ICU 70% of aSAH Cases Result in Death or Permanent Disability Most Critical Unmet Needs Sources: ClearView Analysis (2025). Forian Claims
Data. Becske T. (2018). Steven (2020). Hoh (2023). Etminan. JAMA Neurol. 2019; Fegin. The Lancet Neurology. 2009; Labovitz. Neuroepidemiology. 2006; Shea. Neurosurgery. 2007, Linn. Stroke. 1996; Anderson. Stroke. 2000; Daniere. J de Radiologie
Diagnostique. 2015; Ingall. Stroke. 1989; Giordan et al. J Neurosurg. 2021; Rinkel et al. Lancet Neurol. 2011; Intl Study of Unruptured Intracranial Aneurysms Investigators. NEJM. 1998.
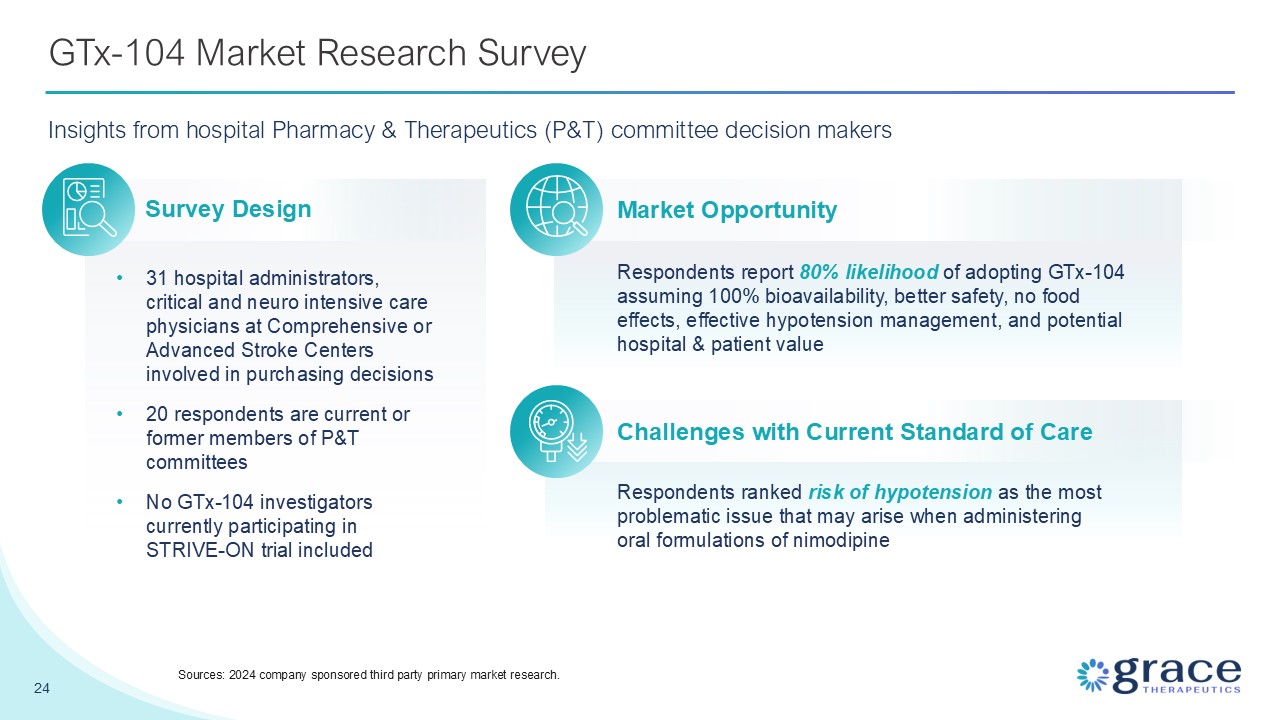
GTx-104 Market Research Survey Insights from hospital Pharmacy & Therapeutics
(P&T) committee decision makers 31 hospital administrators, critical and neuro intensive care physicians at Comprehensive or Advanced Stroke Centers involved in purchasing decisions 20 respondents are current or former members of P&T
committees No GTx-104 investigators currently participating in STRIVE-ON trial included Respondents report 80% likelihood of adopting GTx-104 assuming 100% bioavailability, better safety, no food effects, effective hypotension management, and
potential hospital & patient value Respondents ranked risk of hypotension as the most problematic issue that may arise when administering oral formulations of nimodipine Survey Design Market Opportunity Challenges with Current Standard
of Care 24 Sources: 2024 company sponsored third party primary market research.
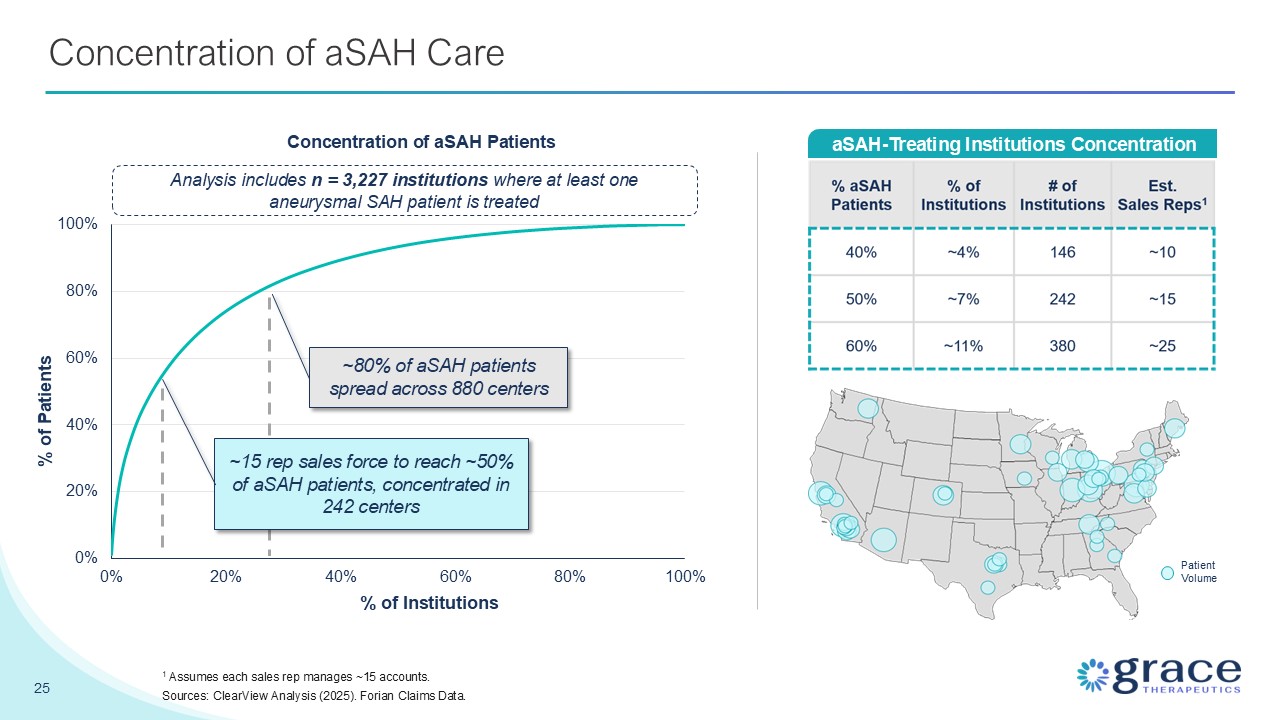
Concentration of aSAH Care 25 aSAH-Treating Institutions
Concentration PatientVolume Analysis includes n = 3,227 institutions where at least one aneurysmal SAH patient is treated Concentration of aSAH Patients % of Institutions % of Patients ~80% of aSAH patients spread across 880 centers ~15
rep sales force to reach ~50% of aSAH patients, concentrated in 242 centers 1 Assumes each sales rep manages ~15 accounts. Sources: ClearView Analysis (2025). Forian Claims Data.
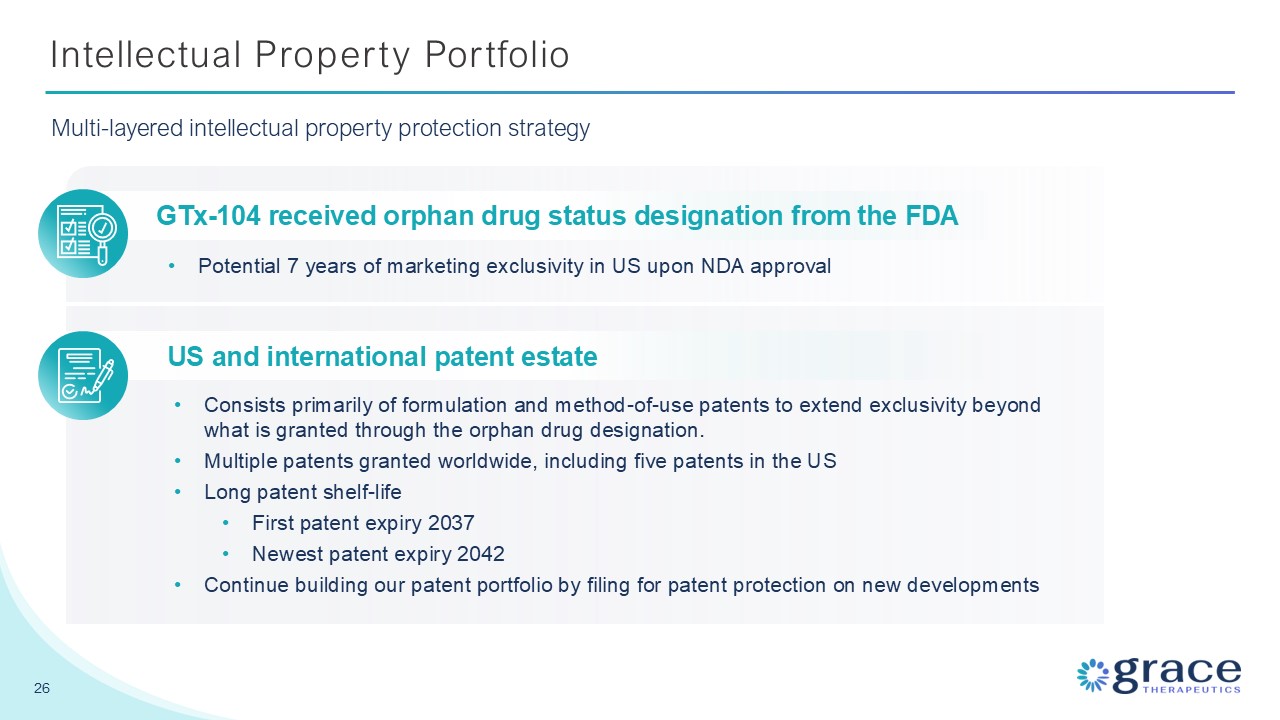
Intellectual Property Portfolio 26 Multi-layered intellectual property
protection strategy GTx-104 received orphan drug status designation from the FDA Potential 7 years of marketing exclusivity in US upon NDA approval US and international patent estate Consists primarily of formulation and method-of-use
patents to extend exclusivity beyond what is granted through the orphan drug designation. Multiple patents granted worldwide, including five patents in the US Long patent shelf-life First patent expiry 2037 Newest patent expiry
2042 Continue building our patent portfolio by filing for patent protection on new developments
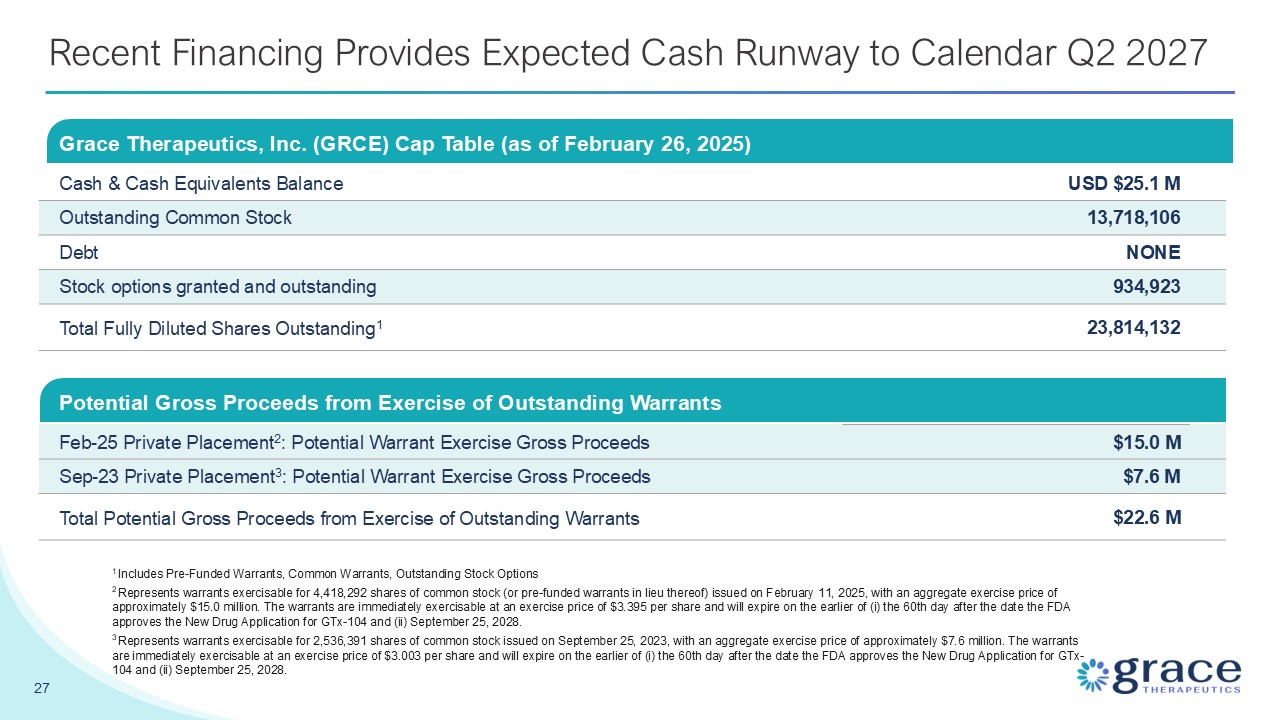
Recent Financing Provides Expected Cash Runway to Calendar Q2 2027 Grace
Therapeutics, Inc. (GRCE) Cap Table (as of February 26, 2025) Cash & Cash Equivalents Balance USD $25.1 M Outstanding Common Stock 13,718,106 Debt NONE Stock options granted and outstanding 934,923 Total Fully Diluted Shares
Outstanding1 23,814,132 27 1 Includes Pre-Funded Warrants, Common Warrants, Outstanding Stock Options 2 Represents warrants exercisable for 4,418,292 shares of common stock (or pre-funded warrants in lieu thereof) issued on February 11,
2025, with an aggregate exercise price of approximately $15.0 million. The warrants are immediately exercisable at an exercise price of $3.395 per share and will expire on the earlier of (i) the 60th day after the date the FDA approves the New
Drug Application for GTx-104 and (ii) September 25, 2028. 3 Represents warrants exercisable for 2,536,391 shares of common stock issued on September 25, 2023, with an aggregate exercise price of approximately $7.6 million. The warrants are
immediately exercisable at an exercise price of $3.003 per share and will expire on the earlier of (i) the 60th day after the date the FDA approves the New Drug Application for GTx-104 and (ii) September 25, 2028. Potential Gross Proceeds from
Exercise of Outstanding Warrants Feb-25 Private Placement2: Potential Warrant Exercise Gross Proceeds $15.0 M Sep-23 Private Placement3: Potential Warrant Exercise Gross Proceeds $7.6 M Total Potential Gross Proceeds from Exercise of
Outstanding Warrants $22.6 M

Appendix (Deprioritized Programs)
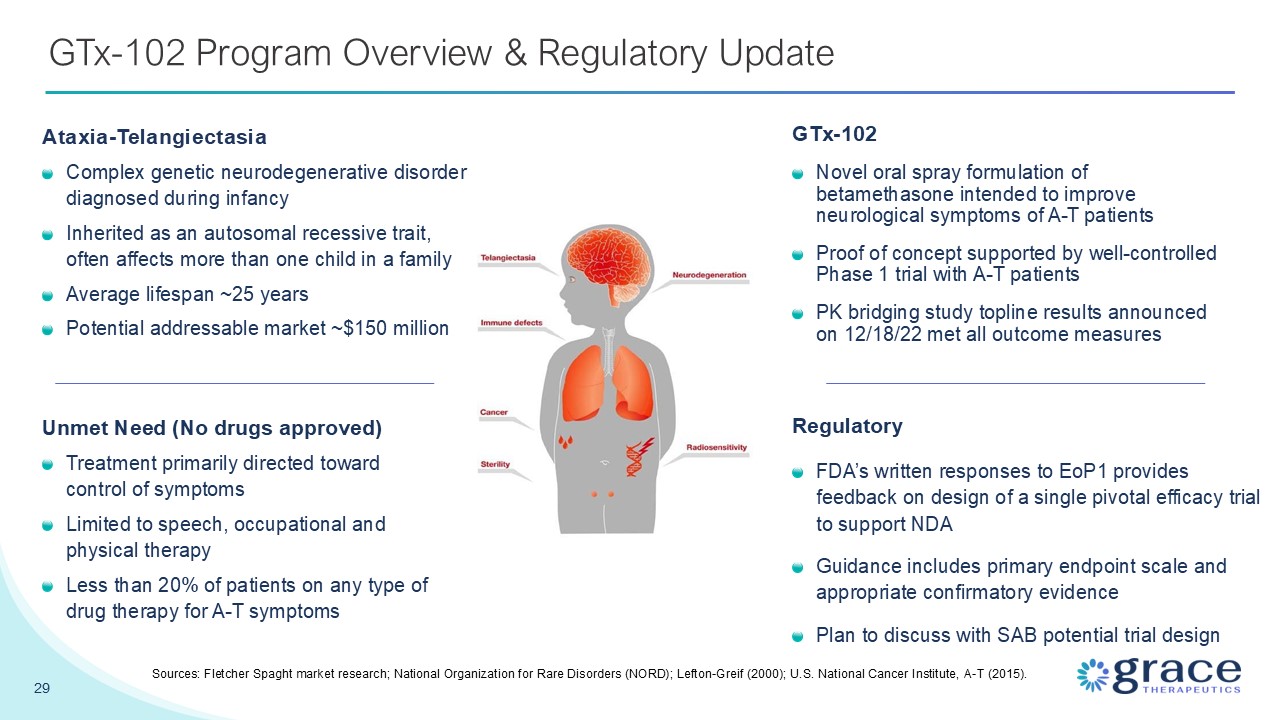
29 GTx-102 Program Overview & Regulatory Update GTx-102 Novel oral spray
formulation of betamethasone intended to improve neurological symptoms of A-T patients Proof of concept supported by well-controlled Phase 1 trial with A-T patients PK bridging study topline results announced on 12/18/22 met all outcome
measures Sources: Fletcher Spaght market research; National Organization for Rare Disorders (NORD); Lefton-Greif (2000); U.S. National Cancer Institute, A-T (2015). Unmet Need (No drugs approved) Treatment primarily directed toward control
of symptoms Limited to speech, occupational and physical therapy Less than 20% of patients on any type of drug therapy for A-T symptoms Ataxia-Telangiectasia Complex genetic neurodegenerative disorder diagnosed during infancy Inherited as
an autosomal recessive trait, often affects more than one child in a family Average lifespan ~25 years Potential addressable market ~$150 million Regulatory FDA’s written responses to EoP1 provides feedback on design of a single pivotal
efficacy trial to support NDA Guidance includes primary endpoint scale and appropriate confirmatory evidence Plan to discuss with SAB potential trial design

30 GTx-101 Program Overview GTx-101 Non-narcotic, topical, bio-adhesive,
transparent film-forming bupivacaine spray Biphasic drug release expected to provide immediate and continuous relief Potential Addressable market ~$200m (PHN) to ~$2.5b (lidocaine patch replacement) PHN: Postherpetic Neuralgia Sources:
Fletcher Spaght, Inc. analysis (2022); CDC MMWR June 6, 2008. UK and several US states have reclassified gabapentin as a scheduled drug Unmet Need Oral therapies (gabapentin, anticonvulsants, opioids) can have side effects and insufficient to
manage pain in many cases Can be prone to abuse Lidocaine patches are hard to place, can cause skin irritation, are 12-hour on / off ~40% experience insufficient pain relief Postherpetic Neuralgia (rare disease) Caused by nerve damage
from the herpes zoster virus which causes shingles Burning, painful, itchy, loss of feeling, sensitivity to touch or temperature, feeling worn out Symptoms can last for several years or may be permanent Regulatory Completed Phase 1
(single dose) in 2022 Met all primary outcome measures Clinical roadmap includes Phase 1 (multiple ascending dose) and Phase 2 (POC)