10-K: Annual report pursuant to Section 13 and 15(d)
Published on June 21, 2024
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
(Mark One)
For the fiscal year ended
or
For the transition period from |
|
to |
|
Commission file number:
(Exact name of registrant as specified in its charter)
Québec, |
|
|
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification Number) |
(Address of principal executive offices, including zip code)
Registrant’s telephone number, including area code:
Securities registered pursuant to Section 12(b) of the Act:
Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes ☐
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer |
☐ |
Accelerated filer |
☐ |
|
|
|
|
☒ |
Smaller reporting company |
||
|
|
|
|
|
|
Emerging growth company |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. Yes ☐ No
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements.
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
☐ Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Securities Exchange Act of 1934). Yes ☐ No
The aggregate market value of the voting and non-voting common shares held by non-affiliates of the registrant, based on the closing sale price of the registrant’s common shares on the last business day of its most recently completed second fiscal quarter, as reported on the Nasdaq Stock Market LLC, was approximately $
Auditor Firm Id:
Former Auditor Firm Id: 1263 Auditor Name: Ernst & Young LLP Auditor Location: Montréal, QC, Canada
ACASTI PHARMA INC.
FORM 10-K
For the Fiscal Year Ended March 31, 2024
Table of Contents
|
|
|
|
|
Item 1. |
9 |
|
|
Item 1A. |
29 |
|
|
Item 1B. |
46 |
|
|
Item 2. |
47 |
|
|
Item 3. |
47 |
|
|
Item 4. |
47 |
|
|
|
|
|
|
Item 5. |
48 |
|
|
Item 6. |
53 |
|
|
Item 7. |
Management’s Discussion and Analysis of Financial Condition and Results of Operation |
54 |
|
Item 7A. |
63 |
|
|
Item 8. |
63 |
|
|
Item 9. |
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
63 |
|
Item 9A. |
63 |
|
|
Item 9B. |
63 |
|
|
Item 9C. |
Disclosure Regarding Foreign Jurisdictions that Prevent Inspections |
|
|
|
|
|
|
Item 10. |
64 |
|
|
Item 11. |
68 |
|
|
Item 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Shareholder Matters |
77 |
|
Item 13. |
Certain Relationships and Related Transactions and Director Independence |
80 |
|
Item 14. |
83 |
|
|
|
|
|
|
Item 15. |
85 |
|
|
Item 16. |
86 |
|
|
|
||
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains information that may be forward-looking information within the meaning of Canadian securities laws and forward-looking statements within the meaning of U.S. federal securities laws, both of which we refer to in this Annual Report on Form 10-K as forward-looking information. Forward-looking information can be identified by the use of terms such as “may”,” “will”,” “should”,” “expect”,” “plan”,” “anticipate”,” “believe”,” “intend”,” “estimate”,” “predict”,” “potential”,” “continue” or other similar expressions concerning matters that are not statements about the present or historical facts. Forward-looking information in this Annual Report on Form 10-K includes, among other things, information or statements about:
In addition, the forward-looking statements in this Annual Report on Form 10-K are subject to a number of known and unknown risks, uncertainties and other factors many of which are beyond our control, that could cause our actual results and developments to differ materially from those that are disclosed in or implied by the forward-looking statements, including, among others:
All of the forward-looking statements in this Annual Report on Form 10-K are qualified by this cautionary statement. There can be no guarantee that the results or developments that we anticipate will be realized or, even if substantially realized, that they will have the consequences or effects on our business, financial condition, or results of operations that we anticipate. As a result, you should not place undue reliance on the forward-looking statements. Except as required by applicable law, we do not undertake to update or amend any forward-looking statements, whether as a result of new information, future events or otherwise. All forward-looking statements are made as of the date of this annual report.
We express all amounts in this Annual Report on Form 10-K in U.S. dollars, except where otherwise indicated. References to “$” and “U.S.$” are to U.S. dollars and references to “C$” or “CAD$” are to Canadian dollars.
Except as otherwise indicated, references in this Annual Report on Form 10-K to “Acasti,” “the Company,” “we,” “us” and “our” refer to Acasti Pharma Inc. and its consolidated subsidiaries.
Risk Factor Summary
The risk factors summarized below could materially harm our business, operating results and/or financial condition, impair our future prospects and/or cause the price of our common shares to decline. For more information, see “Item 1A. Risk Factors” in this Form 10-K. Material risks that may affect our business, operating results and financial condition include, but are not necessarily limited to, the following:
Risk Factors Relating to our Business
Risks Related to Development, Testing and Commercialization of Our Products
Risks Relating to our Intellectual Property
Risks Related to Our Dependence on Third Parties
Risks Related to Tax
Risks Relating to Ownership of our Common Shares
Note Regarding Reverse Stock Split
The Company effected a reverse stock split of its authorized and issued Class A common shares, no par value per share (the “Common Shares), at a ratio of 1-for-6, effective as of July 10, 2023, for the purpose of complying with Nasdaq Listing Rule 5550(a)(2). We have reflected the reverse stock split herein, unless otherwise indicated.
PART I
Item 1. Business
Overview
We are focused on developing and commercializing products for rare and orphan diseases that have the potential to improve clinical outcomes by using our novel drug delivery technologies. We seek to apply new proprietary formulations to approved and marketed pharmaceutical compounds to achieve enhanced efficacy, faster onset of action, reduced side effects, more convenient drug delivery and increased patient compliance; all of which could result in improved patient outcomes. The active pharmaceutical ingredients used in the drug candidates under development by Acasti may be already approved in a target indication or could be repurposed for use in new indications.
The existing well understood efficacy and safety profiles of these marketed compounds provides the opportunity for us to utilize the Section 505(b)(2) regulatory pathway under the Federal Food, Drug and Cosmetic Act (“FDCA”) for the development of our reformulated versions of these drugs, and therefore may provide a potentially shorter path to regulatory approval. Under Section 505(b)(2), if sufficient support of a product’s safety and efficacy either through previous U.S. Food and Drug Administration ("FDA") experience or sufficiently within the existing and accepted scientific literature, can be established, it may eliminate the need to conduct some of the pre-clinical studies and clinical trials that new drug candidates might otherwise require.
Our therapeutic pipeline consists of three unique clinical-stage drug candidates supported by an intellectual property portfolio of more than 40 granted and pending patents in various jurisdictions worldwide. These drug candidates aim to improve clinical outcomes in the treatment of rare and orphan diseases by applying proprietary formulation and drug delivery technologies to existing pharmaceutical compounds to achieve improvements over the current standard of care, or to provide treatment for diseases with no currently approved therapies.
We believe that rare disorders represent an attractive area for drug development, and there remains an opportunity for us to utilize already approved drugs that have established safety profiles and clinical experience to potentially address significant unmet medical needs. A key advantage of pursuing therapies for rare disorders is the potential to receive orphan drug designation (“ODD”) from the FDA. Our three drug candidates have received ODD status, provided certain conditions are met at new drug application ("NDA") approval. ODD provides for seven years of marketing exclusivity in the United States post-launch, provided certain conditions are met, and the potential for faster regulatory review. ODD status can also result in tax credits of up to 50% of clinical development costs conducted in the United States upon marketing approval and a waiver of the NDA fees, which we estimate can translate into savings of approximately $3.2 million for our lead drug candidate, GTX-104. Developing drugs for rare diseases can often allow for clinical trials that are more manageably scaled and may require a smaller, more targeted commercial infrastructure.
The specific diseases targeted for drug development by us are well understood, although the patient populations suffering from such diseases may remain poorly served by available therapies or, in some cases, approved therapies do not yet exist. We aim to effectively treat debilitating symptoms that result from these underlying diseases.
9
Our lead drug candidate:
Other pipeline drug candidates:
In May 2023, we announced the strategic decision to prioritize development of GTX-104 with a goal to advance the product candidate to commercialization, while conserving resources as much as possible to complete development efficiently. We estimate that the deferral of GTX-102 and GTX-101 clinical development could be at least three years from April 2023, given the timeline to complete the development and potential commercial launch of GTX-104. Further development of GTX-102 and GTX-101 will occur at such time as we obtain additional funding, or enter into strategic partnerships for license or sale with third parties.
The decision to defer further development of GTX-102 and GTX-101 triggered a comprehensive impairment review of our intangible assets as of March 31, 2023. Given the extended timeline, we increased the discount rates used to value the related assets in order to recognize additional risks related to prioritizing one asset over the others, the financing for the projects given limited available resources and the need to preserve cash to advance GTX-104 as far as possible, potential competitor advances that could arise over three years, the general market depression affecting small cap development companies like us, and the prohibitively high dilution and expense of available funding in the capital markets. Increasing the discount rates significantly reduced the discounted cash flow values for each of the programs deferred. Accordingly, in the year ended March 31, 2023, we recorded impairment charges related to GTX-102 and GTX-101 of $22.7 million and $6.0 million respectively, together with further adjustments made to deferred taxes and goodwill directly related to those assets. The aggregate impairment charge was $33.5 million. We continue to believe that GTX-102 and GTX-101 may eventually provide significant value when development resumes and, if approved, commercialized successfully.
Our management team possesses significant experience in drug formulation and drug delivery research and development, clinical and pharmaceutical development and manufacturing, regulatory affairs, and business development, as well as being well-versed in late-stage drug development and commercialization. Importantly, our team is comprised of industry professionals with deep expertise and knowledge, including a world-renowned practicing neurosurgeon-scientist and respected authority in aSAH, as well as product development, chemistry, manufacturing and controls (“CMC”), planning, implementation, management, and execution of global Phase 2 and Phase 3 trials for GTX-104, and drug commercialization.
GTX-104 Overview
Nimodipine was granted FDA approval in 1988, and is the only approved drug that has been clinically shown to improve neurological outcomes in aSAH patients. It is only available in the United States as a generic oral capsule and as a branded oral liquid solution called NYMALIZE, which is manufactured and sold by Arbor Pharmaceuticals (acquired in September 2021 by Azurity Pharmaceuticals). Nimodipine has poor water solubility and high permeability characteristics as a result of its high lipophilicity. Additionally, orally administered nimodipine has dose-limiting side-effects such as hypotension, poor absorption and low bioavailability resulting from high first-pass metabolism, and a narrow administration window as food effects lower bioavailability significantly. Due to these issues, blood levels of orally administered nimodipine can be highly variable, making it difficult to manage blood pressure in aSAH patients. Nimodipine capsules are also difficult to administer, particularly to unconscious patients or those with impaired ability to swallow. Concomitant use with CYP3A inhibitors is contraindicated (NIMODIPINE Capsule PI).
10
NIMOTOP is an injectable form of nimodipine that is manufactured by Bayer Healthcare. It is approved in Europe and in other regulated markets (but not in the United States). It has limited utility for aSAH patients because of its high organic solvent content, namely 23.7% ethanol and 17% polyethylene glycol 400 (NIMOTOP SmPC).
Key potential benefits of GTX-104 include:
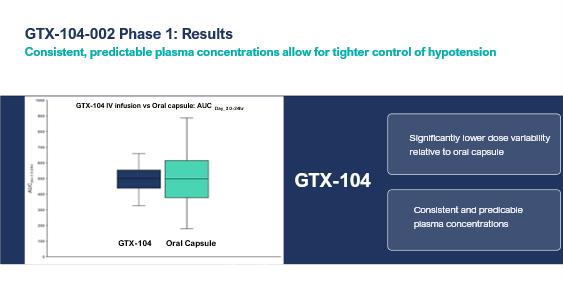
GTX-104 could provide a more convenient mode of administration as compared to generic nimodipine capsules or NYMALIZE. GTX-104 is administered as an IV infusion compared to oral administration via a nasogastric tube in unconscious patients every four hours for both nimodipine capsules and NYMALIZE. Therefore, GTX-104 could make a major contribution to patient care by potentially reducing the dosing associated nursing burden. More convenient, continuous, and consistent dosing can also reduce the risk of medication errors. In addition, as depicted in the charts below, two PK studies conducted by us have shown that GTX-104 has the potential to provide improved bioavailability and show reduced inter- and intra-subject variability compared to oral nimodipine, which is hypothesized to limit the risk of hypotension and to better achieve a desired therapeutic concentration. Following the capsule administration, the variability was observed higher as compared to IV infusion administration (nimodipine exposure variability at steady state observed 37.5% following oral capsule administration versus 15.5%, following GTX-104 IV infusion). Because of its IV formulation, we also expect GTX-104 to reduce certain drug-drug interactions and food effects.
11
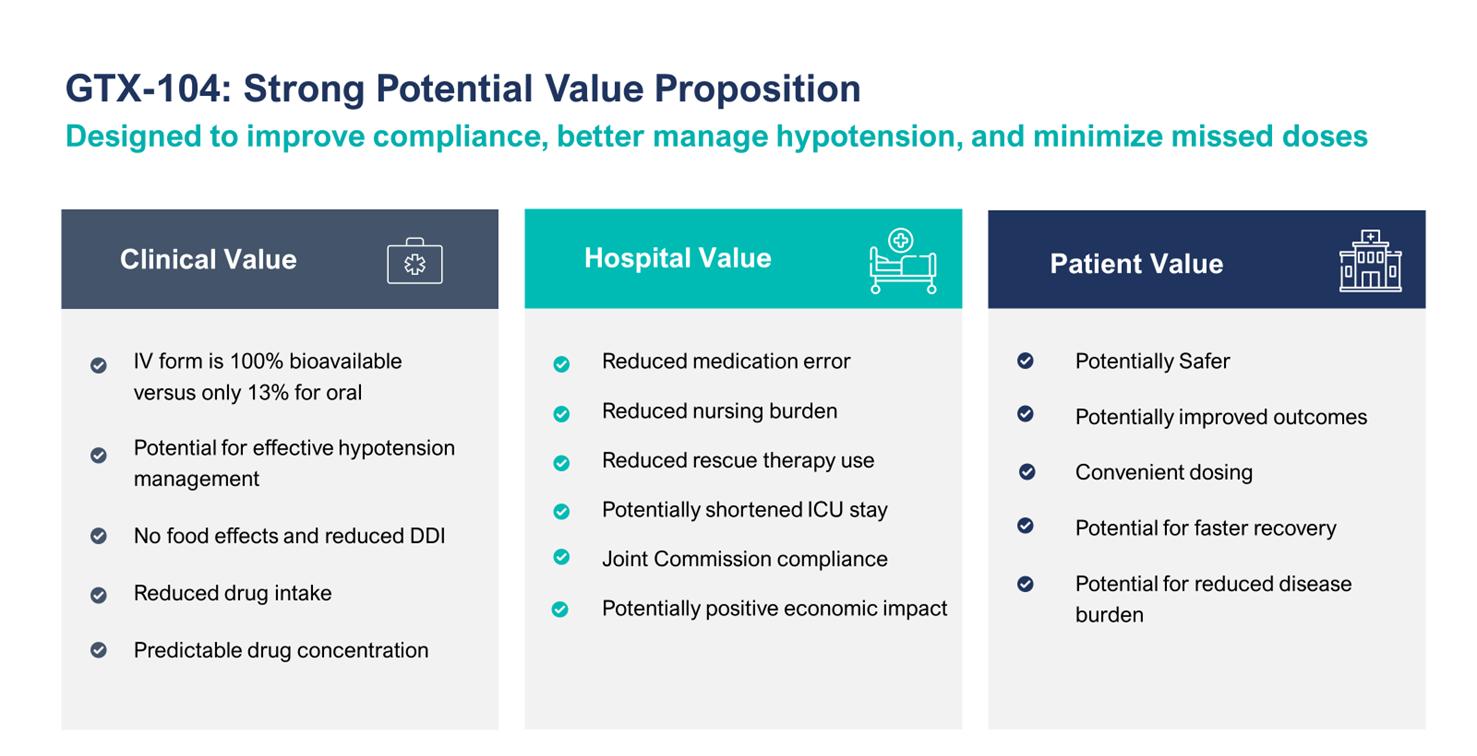
Despite the positive impact it has on recovery, physicians often must discontinue their patients from oral nimodipine, primarily as a result of hypotensive episodes that cannot be controlled by titrating the oral form of drug. Such discontinuation could potentially be avoided by administering GTX-104, which because of its IV administration, may reduce the complexity associated with the need for careful attention to the timing of nimodipine administration at least one hour before or two hours after a meal. Also, unconscious patients will likely receive more consistent concentrations of nimodipine when delivered via the IV route as compared to oral gavage or a nasogastric tube. More consistent dosing is expected to result in a reduction of vasospasm and a better, more consistent management of hypotension. As summarized in the table below, we also anticipate reduced use of rescue therapies, such as vasopressors, and expensive hospital resources, such as the angiography suite, are possible by more effectively managing blood pressure with GTX-104. Reduced incidences of vasospasm could result in shorter length of stay and better outcomes.
About Aneurysmal Subarachnoid Hemorrhage (aSAH)
aSAH is bleeding over the surface of the brain in the subarachnoid space between the brain and the skull, which contains blood vessels that supply the brain. A primary cause of such bleeding is rupture of an aneurysm. The result is a relatively uncommon type of stroke (aSAH) that accounts for about 5% of all strokes and has an incidence of six per 100,000 person years (Becske, 2018).
12
In contrast to more common types of ischemic stroke in elderly individuals, aSAH often occurs at a relatively young age, with approximately half the affected patients younger than 60 years old (Becske, 2018). Approximately 10% to 15% of aSAH patients die before reaching the hospital (Rinkel, 2016), and those who survive the initial hours post hemorrhage are admitted or transferred to tertiary care centers with high risk of complications, including rebleeding and delayed cerebral ischemia (“DCI”). Systemic manifestations affecting cardiovascular, pulmonary, and renal function are common and often complicate management of DCI. Approximately 70% of aSAH patients experience death or a permanent disability, and the mortality rate is about 8.7% at one week, 18.4% at three months, 22.9% at one year, and 29% at five years after the hemorrhage. Of those who survive the initial month, half remain permanently dependent on a caregiver to maintain daily living (Becske, 2018 and Steven 2020).
We estimate that approximately 50,000 individuals experience aSAH each year in the U.S. based on third-party market research, and that total addressable market for aSAH is approximately $300 million in the U.S. There are an estimated 150,000 aSAH patients each year in China and approximately 55,000 patients in the European Union. The unmet needs in the treatment of aSAH and the potential of GTX-104 to address the limitations of the current standard of care were the subject of a Key Opinion Leader event we hosted on October 4, 2023. In an independent market research survey we conducted of hospital administrators, critical and neuro intensive care physicians at institutions with Comprehensive or Advanced Stroke Center certification who are involved in purchasing decisions for their institutions/units, respondents reported 80% likelihood of adopting an IV formulation of nimodipine (GTX-104), assuming 100% bioavailability, better safety, no food effects, effective hypotension management, potential hospital value and patient value.
GTX-104 Development Milestones
In September 2021, we initiated our pivotal PK bridging trial to evaluate the relative bioavailability of GTX-104 compared to currently marketed oral nimodipine capsules in approximately 50 healthy subjects. The PK trial was the next required step in our proposed 505(b)(2) regulatory pathway for GTX-104.
Final results from this pivotal PK trial were reported in May 2022, and showed that the bioavailability of GTX-104 compared favorably with the oral formulation of nimodipine in all subjects, and no serious adverse events were observed for GTX-104.
All endpoints indicated that statistically there was no difference in exposures between GTX-104 and oral nimodipine over the defined time periods for both maximum exposure and total exposure. Plasma concentrations obtained following IV administration showed significantly less variability between subjects as compared to oral administration of capsules, since IV administration is not as sensitive to some of the physiological processes that affect oral administration, such as taking the drug with and without meals, variable gastrointestinal transit time, variable drug uptake from the gastrointestinal tract into the systemic circulation, and variable hepatic blood flow and hepatic first pass metabolism. Previous studies have shown these processes significantly affect the oral bioavailability of nimodipine, and therefore cause oral administration to be prone to larger inter- and intra-subject variability.
The bioavailability of oral nimodipine capsules observed was only ~8% compared to 100% for GTX-104. Consequently, about one-twelfth the amount of nimodipine is delivered with GTX-104 to achieve the same blood levels as with the oral capsules.
No serious adverse events and no adverse events leading to withdrawal were reported during the trial.
Phase 3 STRIVE-ON Randomized Safety Trial for GTX-104
In April 2023, we received a Type C written meeting response and clarifying feedback from the FDA on our proposed pivotal Phase 3 safety trial for GTX-104. The FDA provided additional comments on our development plan that, pending submission of the final clinical protocol and FDA approval, would allow us to proceed with a pivotal Phase 3 safety clinical trial in aSAH patients. On July 5, 2023, we announced the alignment with the FDA on our GTX-104 pivotal Phase 3 safety clinical trial protocol.
The FDA concurred with the suitability of the 505(b)(2) regulatory pathway with the selected Reference Listed Drug NIMOTOP oral capsules ("NDA 018869"), and that our GTX-104-002 PK trial may have met the criteria for a scientific bridge.
The design of our Phase 3 safety clinical trial, which we have titled STRIVE-ON (Safety, Tolerability, Randomized, IV and Oral Nimodipine), is a prospective, open-label, randomized (1:1 ratio), parallel group trial of GTX-104 compared with oral nimodipine, in patients hospitalized for aSAH. Key trial design features include:
13
On October 23, 2023, we enrolled our first patient in our STRIVE-ON clinical trial. Patient enrollment in the STRIVE-ON Phase 3 trial is continuing, and potential NDA submission with the FDA is anticipated to occur in the first half of calendar 2025. We expect this safety trial to be the final clinical step required to seek FDA approval under the 505(b)(2) regulatory pathway.
GTX-102 Overview
GTX-102 is a novel, concentrated oral-mucosal spray of betamethasone intended to improve neurological symptoms of A-T for which there are currently no FDA-approved therapies. GTX-102 is a stable, concentrated oral spray formulation comprised of the gluco-corticosteroid betamethasone that, together with other excipients can be sprayed conveniently over the tongue of the A-T patient and is rapidly absorbed.
About Ataxia Telangiectasia
A-T is a rare genetic progressive autosomal recessive neurodegenerative disorder that affects children, with the hallmark symptoms of cerebellar ataxia and other motor dysfunction, and dilated blood vessels (telangiectasia) that occur in the sclera of the eyes. A-T is caused by mutations in the ataxia telangiectasia gene, which is responsible for modulating cellular response to stress, including breaks in the double strands of DNA.
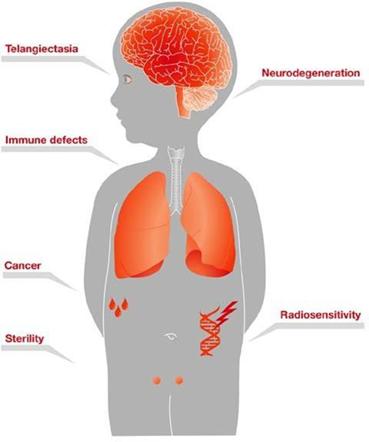
Children with A-T begin to experience balance and coordination problems when they begin to walk (toddler age), and ultimately become wheelchair-bound in their second decade of life. In pre-adolescence (between ages 5 and 8), patients experience oculomotor apraxia, dysarthria, and dysphagia. They also often develop compromised immune systems and are at increased risk of developing respiratory tract infections and cancer (typically lymphomas and leukemia) (U.S. National Cancer Institute A-T, 2015).
A-T is diagnosed through a combination of clinical assessment (especially neurologic and oculomotor deficits), laboratory analysis, and genetic testing. There is no known treatment to slow disease progression, and treatments that are used are strictly aimed at controlling the symptoms (e.g., physical, occupational or speech therapy for neurologic issues), or conditions secondary to the disease (e.g., antibiotics for lung infections, chemotherapy for cancer, etc.) (U.S. National Cancer Institute A-T, 2015). There are no FDA-approved therapeutic options currently available. Patients typically die by age 25 from complications of lung disease or cancer. According to a third-party report we commissioned, A-T affects approximately 4,300 patients per year in the United States and has a potential total addressable market of $150 million, based on the number of treatable patients in the United States.
GTX-102 - R&D and Clinical Trials to Date
We have licensed the data from the multicenter, double-blinded, randomized, placebo-controlled crossover trial from Azienda Ospedaliera Universitaria Senese, Siena, Italy, where Dr. Zannolli et. al. studied the effect of oral liquid solution of betamethasone to
14
reduce ataxia symptoms in patients with A-T. This oral liquid solution is not marketed in the United States, and therefore is not available for clinical use; currently, betamethasone is only available in the United States as an injectable or as a topical cream. This license gives us the right to reference the trial’s data in our NDA filing. On November 12, 2015, we submitted the data from the Zannolli trial to the FDA’s Division of Neurology at a pre-Investigational New Drug (“IND”) meeting and received guidance from the agency on the regulatory requirements to seek approval.
In a multicenter, double-blind, randomized, placebo-controlled crossover trial conducted in Italy, Dr. Zannolli et al. studied the effect of an oral liquid solution of betamethasone on the reduction of ataxia symptoms in 13 children (between ages 2 to 8 years) with A-T. The primary outcome measure was the reduction in ataxia symptoms as assessed by the International Cooperative Ataxia Rating Scale (“ICARS”).
In the trial, oral liquid betamethasone reduced the ICARS total score by a median of 13 points in the intent-to-treat population and 16 points in the per-protocol population (the median percent decreases of ataxia symptoms of 28% and 31%, respectively). Adverse events in the trial were minimal, with no compulsory withdrawals and only minor side effects that did not require medical intervention. Clinical trial results in A-T patients administered oral betamethasone indicated that betamethasone significantly reduced ICARS total score relative to placebo (P = 0.01). The median ICARS change score (change in score with betamethasone minus change in score with placebo) was -13 points (95% confidence interval for the difference in medians was -19 to -5.5 points).
Based on the Zannolli data, we believe that our GTX-102 concentrated oral spray has the potential to provide clinical benefits in decreasing A-T symptoms, including assessments of posture and gait disturbance and kinetic, speech and oculomotor functions. In addition, GTX-102 may ease drug administration for patients experiencing A-T given its application of 1-3x/day of 140µL of concentrated betamethasone liquid sprayed onto the tongue using a more convenient metered dose delivery system, as these A-T patients typically have difficulty swallowing (Lefton-Grief, 2000).
15
GTX-102 PK Data to Date:
GTX-102 administered as a concentrated oral spray achieves similar blood levels at only 1/70th the volume of an oral solution of betamethasone. This more convenient mode of administration will be important for A-T patients who have difficulties swallowing large volumes of liquids.
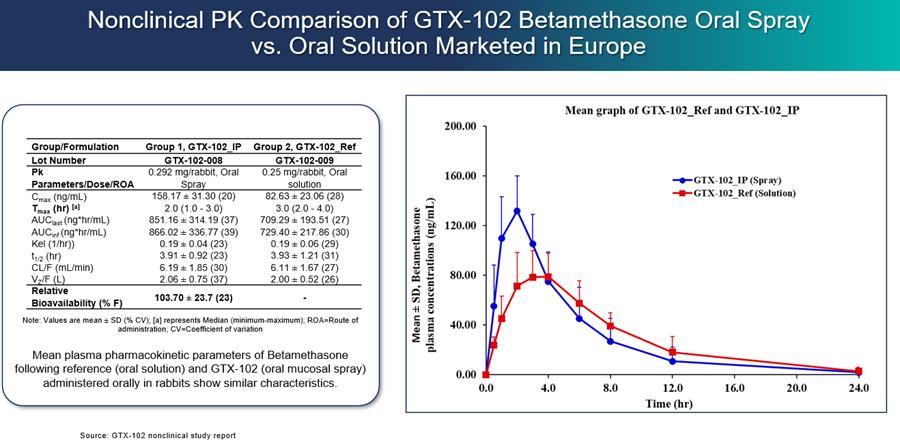
We initiated a PK bridging trial of GTX-102 as compared to the oral liquid solution of betamethasone used in the Zannolli trial and against the injectable form of betamethasone that is approved in the U.S. in the third calendar quarter of 2022. The primary objectives of the PK bridging trial were to evaluate the bioavailability, pharmacokinetics and safety of GTX-102. In December 2022, we reported that the topline results of this trial met all primary outcome measures.
Results showed that GTX-102 betamethasone blood concentrations were highly predictable and consistent based on AUC (the area under the concentration time curve up to 72 hours post-dose, extrapolated to infinity) and Cmax (the maximum concentration occurring between 0 hour to 72 hours after trial drug administration), indicating good linearity and dose-proportionality. GTX-102 betamethasone blood concentrations were within the same range of exposure as IM betamethasone, based on AUC. This IM formulation will serve as a bridge for GTX-102 in the context of the proposed 505(b)(2) regulatory pathway. GTX-102 betamethasone blood concentrations were also within the same range of exposure as Oral Solution ("OS"), based on AUC. This OS formulation was used by Zannolli and may serve as a clinical comparator for further clinical development. Furthermore, statistically there was no significant difference (p>0.05) between GTX-102 administered at a fast rate (each spray immediately following the preceding one) vs. a slow rate (1 spray/minute), as indicated by Cmax and AUC. We believe this result is important because being able to use the fast or the slow rate of administration may provide greater flexibility for patients and caregivers. The Cmax of GTX-102 was within the same range of exposure as the OS, but the Cmax for the IM formulation was lower than both GTX-102 and the OS, as well as what has been reported previously for the IM in industry publications. It is important to note that achieving bioequivalence with the IM was not an objective of this trial, nor was it expected. Finally, of the 48 healthy adult subjects, no serious adverse events were reported, and the most frequent drug-related adverse effect was mild headache (4 cases).
The further clinical development of GTX-102 has been deprioritized in favor of our focus on development of GTX-104. However, we plan to collaborate with clinical experts to design the Phase 3 safety and efficacy protocol for GTX-102 and gain alignment with the FDA on the development path forward to maximize value. Further clinical development work will be contingent on additional funding for GTX-102 or the signing of a strategic partnership. It is also possible that we may out-license or sell our GTX-102 drug candidate.
GTX-101 Overview
16
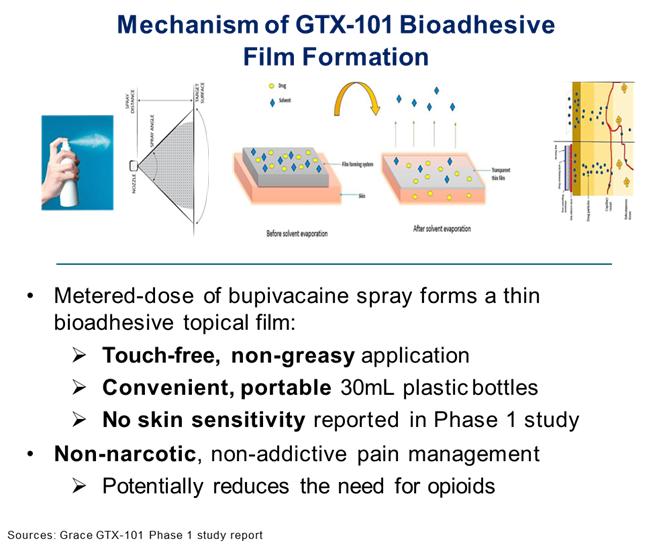
GTX-101 is a non-narcotic, topical bio-adhesive film-forming bupivacaine spray designed to ease the symptoms of patients suffering with postherpetic neuralgia (“PHN”). GTX-101 is administered via a metered-dose of bupivacaine spray and forms a thin bio-adhesive topical film on the surface of the patient’s skin, which enables a touch-free, non-greasy application. It also comes in convenient, portable 30 ml plastic bottles. Unlike oral gabapentin and lidocaine patches which are used for the treatment of PHN, we believe that the biphasic delivery mechanism of GTX-101 has the potential for rapid onset of action and continuous pain relief for up to eight hours. No skin sensitivity was reported in a Phase 1 trial.
About Postherpetic Neuralgia (PHN)
PHN is neuropathic pain due to damage caused by the varicella zoster virus (“VZV”). Infection with VZV causes two distinct clinical conditions. Primary VZV infection causes varicella (i.e., chickenpox), a contagious rash illness that typically occurs among young children. Secondary VZV can reactivate clinically, decades after initial infection, to cause herpes zoster (“HZ”), otherwise known as shingles. Acute HZ arises when dormant virus particles, persisting within an affected sensory ganglion from the earlier, primary infection with VZV become reactivated when cellular immunity to varicella decreases. Viral particles replicate and may spread to the dorsal root, into the dorsal horn of the spinal cord, and through peripheral sensory nerve fibers down to the level of the skin. Viral particles also may circulate in the blood. This reactivation is accompanied by inflammation of the skin, immune response, hemorrhage, and destruction of peripheral and central neurons and their fibers. Following such neural degeneration, distinct types of pathophysiological mechanisms involving both the central and peripheral nervous systems may give rise to the severe nerve pain associated with PHN.
While the rash associated with HZ typically heals within two to four weeks, the pain may persist for months or even years, and this PHN manifestation is the most common and debilitating complication of HZ. There is currently no consensus definition for PHN, but it has been suggested by the Centers for Disease Control and Prevention (“CDC”) that PHN is best defined as pain lasting at least three months after resolution of the rash.
PHN is associated with significant loss of function and reduced quality of life, particularly in the elderly. It has a detrimental effect on all aspects of a patient's quality of life. The nature of PHN pain varies from mild to severe, constant, intermittent, or triggered by trivial stimuli. Approximately half of patients with PHN describe their pain as “horrible” or “excruciating,” ranging in duration from a few minutes to constant on a daily or almost daily basis (Katz, 2004). The pain can disrupt sleep, mood, work, and activities of daily living, adversely impacting the quality of life and leading to social withdrawal and depression. PHN is the number-one cause of intractable, debilitating pain in the elderly, and has been cited as the leading cause of suicide in chronic pain patients over the age of 70 (Hess, 1990).
Current treatment of PHN most often consists of oral gabapentin (first line) and prescription lidocaine patches or antidepressants (second line), and refractory cases may be prescribed opioids to address persistent pain. Gabapentin and opioid abuse have continued to proliferate, and lidocaine patches are suboptimal for many reasons. An independent third-party market research firm we
17
commissioned interviewed more than 250 physicians who regularly treat PHN patients and found that approximately 40% of patients using lidocaine patches experience insufficient pain relief. Lidocaine patches are difficult to use, fall off, and look unsightly with possible skin sensitivity and irritation. Additionally, lidocaine patches can only be used for 12 hours and then need to be removed for 12 hours before being reapplied. Prescription lidocaine patches are only approved for PHN, and the market is currently made up of both branded and generic offerings. It is estimated that PHN affects approximately 120,000 patients per year in the United States. According to the third-party report, the total addressable market for GTX-101 could be as large as $2.5 billion, consisting of approximately $200 million for PHN pain and $2.3 billion for non-PHN pain indications.
GTX-101 R&D History and Clinical Trials Completed to Date
To date, we have conducted four Phase I trials in healthy volunteers to assess the PK, safety and tolerability of GTX-101 and to determine the plasma levels of bupivacaine HCl administered as a single dose in various concentrations between 30 mg (three sprays) and 2100 mg (twenty sprays).
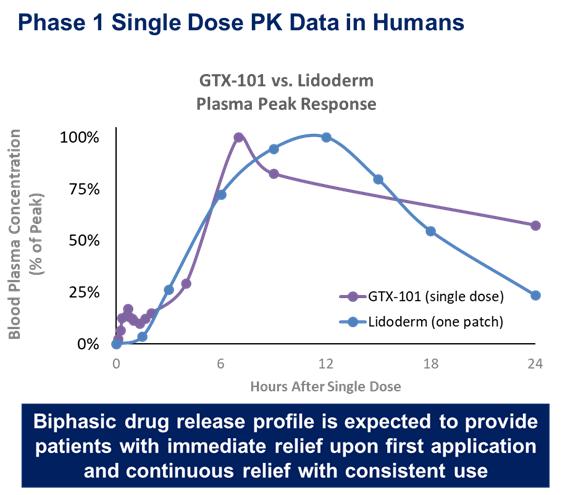
These trials confirmed that bupivacaine delivered as a topical spray (GTX-101) is well absorbed through the skin, as demonstrated in the graph below, while very little is absorbed systemically.
In all four trials, the administration of GTX-101 to healthy volunteers was safe and well tolerated. In addition, no evidence of skin irritation was observed at the application site following the spray administrations. The data below is from two separate trials of GTX-101 and the Lidoderm patch superimposed on each other.
GTX-101 recent activities:
The data from the single dose Phase 1 clinical trial for GTX-101 was submitted to the FDA’s Division of Anesthesiology and feedback was received at a pre-IND meeting that informed the design of pre-clinical toxicology studies and a clinical and regulatory pathway to approval under section 505(b)(2). We completed a minipig skin sensitivity study in the second calendar quarter of 2022, and we initiated a single dose PK trial in healthy human volunteers in July 2022. Topline results from this single dose PK trial were reported in December 2022 and the results met all primary outcome measures.
The median Tmax (the time of maximum concentration between 0 hour and 240 hours after study drug administration) of bupivacaine in plasma following GTX-101 single-dose topical applications ranged between 18 to 24 hours depending on dose, while the median Tmax following the subcutaneous injection of 10 mg of bupivacaine was only 23 minutes. This result suggests that bupivacaine delivered by GTX-101 remains in the skin for a long period of time, potentially inducing prolonged analgesic effect in the sprayed area. The exposure to bupivacaine based on Cmax (the maximum concentration occurring at Tmax between 0 hour and 240 hours after study drug administration) and AUC (the area under the concentration time curve, extrapolated to infinity) following GTX-101 topical application as a single-dose increased with increasing dose.
18
The systemic exposure to bupivacaine following a 200mg dose of GTX-101 was approximately 29-fold less than a single subcutaneous dose of 10mg of bupivacaine based on Cmax and approximately 6-fold less than a single subcutaneous dose of 10mg of bupivacaine based on AUC. We predict these lower blood levels will correspond to an increased safety margin for GTX-101 with regards to toxicity risk. Mean half-life ("T half") following GTX-101 single-dose topical applications ranged between 24 to 37 hours depending on dose, suggesting a slow elimination and potentially long duration of effect, while mean Tmax following the subcutaneous injection of 10 mg of bupivacaine was only 8 hours.
There were only two adverse events judged as related to the study drug by the investigator for each of GTX-101 and the bupivacaine subcutaneous injection. Following GTX-101 topical application: headache (1 event = 3%) and numbness (1 event = 3%) at the sprayed area following bupivacaine subcutaneous injection: dizziness (1 event = 8%) and nausea (1 event = 8%).
The further development of GTX-101 has been deprioritized in favor of our focus on development of GTX-104. Pending additional funding for GTX-101 or the signing of a strategic partnership, we plan to follow this successful PK trial with the next step of the clinical development plan including a multiple ascending dose trial. Results from these non-clinical studies and clinical trials are required before the initiation of our Phase 2 program in PHN patients. It is also possible that we may out-license or sell our GTX-101 drug candidate.
19
Overall Commercialization Strategy
We have worldwide commercialization rights for all our pipeline drug candidates and plan to maximize the value of each of our drug candidates over time. Currently, we have prioritized the development of GTX-104 over that of GTX-102 and GTX-101. If we receive regulatory approval for GTX-104 in the US, we may look to out-license its commercialization or consider self-commercialization including outsourcing sales to ensure efficient commercial management and maximize market penetration and financial returns. We may further seek commercial partnerships to fully exploit the market potential of GTX-104 in territories outside the US. It is possible that we out-license or sell GTX-102 and/or GTX-101 to the US and/or global markets to maximize value.
Manufacturing and Supply
We currently do not own any manufacturing facilities. The manufacture of our pipeline of drug candidates is highly reliant on complex techniques and personnel aseptic techniques, which present significant challenges and require specialized expertise. Further, these processes undergo a high level of scrutiny by regulatory agencies. Consequently, we utilize a network of third-party CMOs for manufacturing our drug candidates. All CMOs are monitored and evaluated by us to assess compliance with regulatory requirements.
We work with independent consultants to perform periodic quality audits of our manufacturers to review the manufacturing process for our drug candidates and to provide input on quality issues. All lots of the drug substance and drug product used in clinical supply are manufactured under current good manufacturing practices. We plan to continue to rely upon CMOs to manufacture clinical and commercial quantities once the product is approved. We have development agreements in place with these CMOs and we have personnel with pharmaceutical development and manufacturing experience who are responsible for the relationships with our CMOs.
Intellectual Property Portfolio
We have a multi-layered intellectual property protection strategy, which we believe will create barriers to entry and solidify our position in the market. All of our clinical pipeline drug candidates have received orphan status designation from the FDA, which could result in 7 years of marketing exclusivity in the United States and 10 years in Europe, provided they receive the final marketing authorizations from the applicable government agencies, and they can meet the conditions for receiving such marketing exclusivity. In addition, we protect our drug candidates through a well-defined patent filing strategy. Our patent estate includes more than 40 granted and pending patents in various global jurisdictions, including 8 U.S. issued patents and 4 filed U.S. patent applications. We believe that our intellectual property portfolio, consisting primarily of composition and method-of-use patents, will protect the market value of our products by extending exclusivity beyond what is granted through the orphan designation. We intend to continue to build our patent portfolio by filing for patent protection on new developments with respect to our product candidates. We expect that these patents will, if and when issued, allow us to list our own patents in the Orange Book: Approved Drug Products with Therapeutic Equivalence issued by the FDA, to which potential competitors will be required to certify upon submission of their applications referencing our drug products, if approved.
We strive to protect and enhance the proprietary technology, inventions, and improvements that are commercially important to the development of our business, including seeking, maintaining, and defending patent rights, whether developed internally or licensed from third parties. We also rely on trade secrets relating to manufacturing know-how, continuing technological innovation and in-licensing opportunities to develop, strengthen, and maintain our proprietary position. We may also rely on regulatory protections afforded through orphan drug status, data exclusivity, market exclusivity, and patent term extensions, where available.
We are actively seeking U.S. and international patent protection for a variety of technologies and intend to seek patent protection or rely upon trade secret rights to protect other technologies that may be used to discover and validate targets and that may be used to identify and develop novel pharmaceutical products. We seek these protections, in part, through confidentiality and proprietary information agreements.
Individual patents extend for varying periods depending on the date of filing or the date of issuance, and the legal term of patents in the countries in which they are obtained. Generally, utility patents issued for applications filed in the United States are granted a term of 20 years from the earliest effective filing date of a non-provisional patent application. In addition, in certain instances, a patent term can be extended to recapture a portion of the U.S. Patent and Trademark Office delay in issuing the patent as well as a portion of the term effectively lost as a result of the FDA regulatory review period. However, as to the FDA component, the restoration period cannot be longer than 5 years and the total patent term including the restoration period must not exceed 14 years following FDA approval. The duration of foreign patents varies in accordance with provisions of applicable local law, but typically is also 20 years from the earliest effective filing date. The actual protection afforded by a patent may vary on a product-by-product basis from country to country and can depend upon many factors, including the type of patent, the scope of its coverage, the availability of regulatory-related extensions, the availability of legal remedies in a particular country and the validity and enforceability of the patent.
20
We have several issued U.S. patents and patent applications as well as patents and patent applications in other jurisdictions. Five patents for GTX-104 have been granted in the United States. One patent for GTX-101 has been granted in Europe, China, Mexico, Japan and South Africa. One patent for GTX-102 has been granted in Japan.
Government Regulation
We are subject to extensive regulation by the FDA and other federal, state, and local regulatory agencies. The FDCA and the FDA's implementing regulations set forth, among other things, requirements for the testing, development, manufacture, quality control, safety, effectiveness, approval, labeling, storage, record-keeping, reporting, distribution, import, export, sale, advertising and promotion of our product candidates. Although we focus on regulation in the U.S., because that is currently our primary focus, we may seek approval for, and market, our products candidates in other countries in the future. Generally, our activities in other countries will be subject to regulation that is similar in nature and scope as that imposed in the U.S., although there can be important differences.
Development and Approval
Under the FDCA, FDA approval of an NDA is required before any new drug can be marketed in the U.S. NDAs require extensive studies and submission of a large amount of data by the applicant.
Preclinical Testing. Before testing any compound in human patients in the U.S., a company must generate extensive preclinical data. Preclinical testing generally includes laboratory evaluation of product chemistry and formulation, as well as toxicological and pharmacological studies in several animal species to assess the toxicity and dosing of the product. Certain animal studies must be performed in compliance with the FDA's Good Laboratory Practice (“GLP”) regulations and the U.S. Department of Agriculture's Animal Welfare Act.
IND Application. Human clinical trials in the U.S. cannot commence until an IND application is submitted and becomes effective. A company must submit preclinical testing results to the FDA as part of the IND, and the FDA must evaluate whether there is an adequate basis for testing the drug in initial clinical studies in human volunteers. Unless the FDA raises concerns, the IND becomes effective 30 days following its receipt by the FDA, and the clinical trial proposed in the IND may begin. Once human clinical trials have commenced, the FDA may stop a clinical trial by placing it on "clinical hold" because of concerns about the safety of the product being tested, or for other reasons.
Clinical Trials. Clinical trials involve the administration of a drug to healthy human volunteers or to patients, under the supervision of a qualified investigator. The conduct of clinical trials is subject to extensive regulation, including compliance with the FDA's bioresearch monitoring regulations and current Good Clinical Practice (“cGCP”) requirements, which establish standards for conducting, recording data from, and reporting the results of, clinical trials, and are intended to assure that the data and reported results are credible and accurate, and that the rights, safety, and well-being of study participants are protected. Clinical trials must be conducted under protocols that detail the study objectives, parameters for monitoring safety, and the efficacy criteria, if any, to be evaluated. Each protocol is reviewed by the FDA as part of the IND. In addition, each clinical trial must be reviewed and approved by, and conducted under the auspices of, an Institutional Review Board (“IRB”) for each clinical site. Companies sponsoring the clinical trials, investigators, and IRBs also must comply with, as applicable, regulations and guidelines for obtaining informed consent from the study patients, following the protocol and investigational plan, adequately monitoring the clinical trial, and timely reporting of adverse events. Foreign studies conducted under an IND must meet the same requirements that apply to studies being conducted in the U.S. Data from a foreign study not conducted under an IND may be submitted in support of an NDA if the study was conducted in accordance with cGCP and the FDA is able to validate the data.
A study sponsor is required to publicly post specified details about certain clinical trials and clinical trial results on government or independent websites (e.g., http://clinicaltrials.gov). Human clinical trials typically are conducted in three sequential phases, although the phases may overlap, be combined, or be subdivided in some cases:
21
The sponsoring company, the FDA, or the IRB may suspend or terminate a clinical trial at any time on various grounds, including a finding that the patients are being exposed to an unacceptable health risk. Further, success in early-stage clinical trials does not assure success in later-stage clinical trials. Data obtained from clinical activities are not always conclusive and may be subject to alternative interpretations that could delay, limit or prevent regulatory approval.
NDA Submission and Review. The FDCA provides two pathways for the approval of new drugs through an NDA. An NDA under Section 505(b)(1) of the FD&C Act is a comprehensive application to support approval of a product candidate that includes, among other things, data and information to demonstrate that the proposed drug is safe and effective for its proposed uses, that production methods are adequate to ensure its identity, strength, quality, and purity of the drug, and that proposed labeling is appropriate and contains all necessary information. A 505(b)(1) NDA contains results of the full set of preclinical studies and clinical trials conducted by or on behalf of the applicant to characterize and evaluate the product candidate.
Section 505(b)(2) of the FDCA provides an alternate regulatory pathway to obtain FDA approval that permits the filing of an NDA where at least some of the information required for approval comes from studies not conducted by or for the applicant and for which the applicant has not obtained a right of reference. The applicant may rely to some extent upon the FDA's findings of safety and effectiveness for an approved product that acts as the reference drug and submit its own product-specific data — which may include data from preclinical studies or clinical trials conducted by or on behalf of the applicant — to address differences between the product candidate and the reference drug. We are pursuing the Section 505(b)(2) regulatory approval pathway for GTX-104, with NIMOTOP oral capsules as the reference drug.
The submission of an NDA under either Section 505(b)(1) or Section 505(b)(2) generally requires payment of a substantial user fee to the FDA. However, such fees can be waived by the FDA for orphan drugs such as GTX-104.
The FDA reviews applications to determine, among other things, whether a product is safe and effective for its intended use and whether the manufacturing controls are adequate to assure and preserve the product's identity, strength, quality, and purity. For some NDAs, the FDA may convene an advisory committee to seek insights and recommendations on issues relevant to approval of the application. Although the FDA is not bound by the recommendation of an advisory committee, the agency considers such recommendations carefully when making decisions.
The FDA may determine that a Risk Evaluation and Mitigation Strategy (“REMS”) is necessary to ensure that the benefits of a new product outweigh its risks, and the product can therefore be approved. A REMS may include various elements, ranging from a medication guide or patient package insert to limitations on who may prescribe or dispense the drug, depending on what the FDA considers necessary for the safe use of the drug. Under the Pediatric Research Equity Act, certain applications for approval must also include an assessment, generally based on clinical study data, of the safety and effectiveness of the subject drug in relevant pediatric populations. Before approving an NDA, the FDA will inspect the facility or facilities where the product is manufactured. The FDA will not approve an application unless it determines that the manufacturing processes and facilities are compliant with current Good Manufacturing Practice (“cGMP”) requirements and adequate to assure consistent production of the product within required specifications.
Once the FDA accepts an NDA submission which occurs, if at all, within 60 days after submission of the NDA – the FDA's goal for a non-priority review of an NDA is ten months. The review process can be and often is significantly extended, however, by FDA requests for additional information, studies, or clarification. After reviewing an NDA and the facilities where the product is manufactured, the FDA either issues an approval letter or a complete response letter (“CRL”) outlining the deficiencies in the submission. The CRL may require additional testing or information, including additional preclinical or clinical data. Even if such additional information and data are submitted, the FDA may decide that the NDA still does not meet the standards for approval. Data from clinical trials are not always conclusive and the FDA may interpret data differently than the sponsor.
Obtaining regulatory approval often takes several years, involves the expenditure of substantial resources, and depends on several factors, including the severity of the disease in question, the availability of alternative treatments, and the risks and benefits demonstrated in clinical trials. Additionally, as a condition of approval, the FDA may impose restrictions that could affect the commercial success of a drug or require post-approval commitments, including the completion within a specified time of additional clinical studies, which often are referred to as "Phase 4" or "post-marketing" studies.
22
Post-approval modifications to the drug, such as changes in indications, labeling, or manufacturing processes or facilities, may require a sponsor to develop additional data or conduct additional preclinical studies or clinical trials, to be submitted in a new or supplemental NDA, which would require FDA approval.
Post-Approval Regulation
Once approved, drug products are subject to continuing regulation by the FDA. If ongoing regulatory requirements are not met or if safety or manufacturing problems occur after the product reaches the market, the FDA may at any time withdraw product approval or take actions that would limit or suspend marketing. Additionally, the FDA may require post-marketing studies or clinical trials, changes to a product’s approved labeling, including the addition of new warnings and contraindications, or the implementation of other risk management measures, including distribution-related restrictions, if there are new safety information developments.
Good Manufacturing Practices. Companies engaged in manufacturing drug products, or their components must comply with applicable cGMP requirements and product-specific regulations enforced by the FDA and other regulatory agencies. Compliance with cGMP includes adhering to requirements relating to organization and training of personnel, buildings and facilities, equipment, control of components and drug product containers and closures, production and process controls, quality control and quality assurance, packaging and labeling controls, holding and distribution, laboratory controls, and records and reports. The FDA regulates and inspects equipment, facilities, and processes used in manufacturing pharmaceutical products prior to approval. If, after receiving approval, a company makes a material change in manufacturing equipment, location, or process (all of which are, to some degree, incorporated in the NDA), additional regulatory review and approval may be required. The FDA also conducts regular, periodic visits to re-inspect equipment, facilities, and processes following the initial approval of a product. Failure to comply with applicable cGMP requirements and conditions of product approval may lead the FDA to take enforcement action or seek sanctions, including fines, issuance of warning letters, civil penalties, injunctions, suspension of manufacturing operations, operating restrictions, withdrawal of FDA approval, seizure or recall of products, and criminal prosecution. Although we periodically monitor the FDA compliance of our third-party manufacturers, we cannot be certain that our present or future third-party manufacturers will consistently comply with cGMP and other applicable FDA regulatory requirements.
Advertising and Promotion. The FDA and other federal regulatory agencies closely regulate the marketing and promotion of drugs through, among other things, standards and regulations for direct-to-consumer advertising, advertising and promotion to healthcare professionals, communications regarding unapproved uses, industry-sponsored scientific and educational activities, and promotional activities involving the Internet. A product cannot be commercially promoted before it is approved. After approval, product promotion can include only those claims relating to safety and effectiveness that are consistent with the labeling approved by the FDA. Healthcare providers are permitted to prescribe drugs for "off-label" uses – that is, uses not approved by the FDA and not described in the product's labeling – because the FDA does not regulate the practice of medicine. However, FDA regulations impose restrictions on manufacturers' communications regarding off-label uses. Broadly speaking, a manufacturer may not promote a drug for off-label use, but under certain conditions may engage in non-promotional, balanced, scientific communication regarding off-label use. In addition to FDA restrictions on marketing of pharmaceutical products, state and federal fraud and abuse laws have been applied to restrict certain marketing practices in the pharmaceutical industry. Failure to comply with applicable FDA requirements and restrictions in this area may subject a company to adverse publicity and enforcement action by the FDA, the Department of Justice, or the Office of the Inspector General of the Department of Health and Human Services, as well as state authorities. This could subject a company to a range of penalties that could have a significant commercial impact, including civil and criminal fines and agreements that materially restrict the way a company promotes or distributes a drug.
Other Requirements. NDA holders must comply with other regulatory requirements, including submitting annual reports, reporting information about adverse drug experiences, and maintaining certain records.
Hatch-Waxman Act
The Drug Price Competition and Patent Term Restoration Act of 1984 (the “Hatch-Waxman Act”) establishes two abbreviated approval pathways for pharmaceutical products that are in some way follow-on versions of already approved products.
Generic Drugs. A generic version of an approved drug is approved by means of abbreviated NDA (“ANDA”), by which the sponsor demonstrates that the proposed product is the same as the approved, brand-name drug, which is referred to as the reference listed drug (“RLD”). Generally, an ANDA must contain data and information showing that the proposed generic product and RLD (i) have the same active ingredient, in the same strength and dosage form, to be delivered via the same route of administration, (ii) are intended for the same uses, and (iii) are bioequivalent. This is instead of independently demonstrating the proposed product's safety and effectiveness, which are inferred from the fact that the product is the same as the RLD, which the FDA previously found to be safe and effective.
23
505(b)(2) NDAs. As discussed above, if a product is similar, but not identical, to an already approved product, it may be submitted for approval via an NDA under section 505(b)(2) of the FDCA. Unlike ANDA, this does not excuse the sponsor from demonstrating the proposed product's safety and effectiveness. Rather, the sponsor is permitted to rely to some degree on information from investigations that were not conducted by or for the applicant and for which the applicant has not obtained a right of reference and must submit its own product-specific data of safety and effectiveness to an extent necessary because of the differences between the products. An NDA approved under 505(b)(2) may in turn serve as an RLD for subsequent applications from other sponsors.
RLD Patents. In an NDA, a sponsor must identify patents that claim the drug substance or drug product or a method of using the drug. When the drug is approved, those patents are among the information about the product that is listed in the FDA publication, Approved Drug Products with Therapeutic Equivalence Evaluations, which is referred to as the Orange Book. The sponsor of an ANDA or 505(b)(2) application seeking to rely on an approved product as the RLD must make one of several certifications regarding each listed patent. A “Paragraph I” certification is the sponsor’s statement that patent information has not been filed for the RLD. A “Paragraph II” certification is the sponsor’s statement that the RLD’s patents have expired. A "Paragraph III" certification is the sponsor's statement that it will wait for the patent to expire before obtaining approval for its product. A "Paragraph IV" certification is an assertion that the patent does not block approval of the later product, either because the patent is invalid or unenforceable or because the patent, even if valid, is not infringed by the new product.
Regulatory Exclusivities. The Hatch-Waxman Act provides periods of regulatory exclusivity for products that would serve as RLDs for an ANDA or 505(b)(2) application. If a product is a "new chemical entity," or “NCE” — generally meaning that the active moiety has never before been approved in any drug — there is a period of five years from the product's approval during which the FDA may not accept for filing any ANDA or 505(b)(2) application for a drug with the same active moiety. An ANDA or 505(b)(2) application may be submitted after four years, however, if the sponsor of the application makes a Paragraph IV certification.
A product that is not an NCE may qualify for a three-year period of exclusivity if the NDA contains new clinical data, (other than bioavailability studies) derived from studies conducted by or for the sponsor, that were necessary for approval. In that instance, the exclusivity period does not preclude filing or review of an ANDA or 505(b)(2) application; rather, the FDA is precluded from granting final approval to the ANDA or 505(b)(2) application until three years after approval of the RLD. Additionally, the exclusivity applies only to the conditions of approval that require submission of the clinical data.
Once the FDA accepts for filing an ANDA or 505(b)(2) application containing a Paragraph IV certification, the applicant must within 20 days provide notice to the RLD or listed drug NDA holder and patent owner that the application has been submitted and provide the factual and legal basis for the applicant's assertion that the patent is invalid or not infringed. If the NDA holder or patent owner files suit against the ANDA or 505(b)(2) applicant for patent infringement within 45 days of receiving the Paragraph IV notice, the FDA is prohibited from approving the ANDA or 505(b)(2) application for a period of 30 months or the resolution of the underlying suit, whichever is earlier. If the RLD has NCE exclusivity and the notice is given and suit filed during the fifth year of exclusivity, the regulatory stay extends until 7.5 years after the RLD approval. The FDA may approve the proposed product before the expiration of the regulatory stay if a court finds the patent invalid or not infringed or if the court shortens the period because the parties have failed to cooperate in expediting the litigation.
If the RLD has NCE exclusivity and the notice is given and suit filed during the fifth year of exclusivity, the regulatory stay extends until 7.5 years after the RLD approval. The FDA may approve the proposed product before the expiration of the regulatory stay if a court finds the patent invalid or not infringed or if the court shortens the period because the parties have failed to cooperate in expediting the litigation.
Patent Term Restoration. A portion of the patent term lost during product development and FDA review of an NDA is restored if approval of the application is the first permitted commercial marketing of a drug containing the active ingredient. The patent term restoration period is generally one-half the time between the effective date of the IND or the date of patent grant (whichever is later) and the date of submission of the NDA, plus the time between the date of submission of the NDA and the date of FDA approval of the product. The maximum period of restoration is five years, and the patent cannot be extended to more than 14 years from the date of FDA approval of the product. Only one patent claiming each approved product is eligible for restoration and the patent holder must apply for restoration within 60 days of approval. The U.S. Patent and Trademark Office in consultation with the FDA, reviews and approves the application for patent term restoration.
Other Exclusivities
24
Pediatric Exclusivity. Section 505A of the FDCA provides for six months of additional exclusivity or patent protection if an NDA sponsor submits pediatric data that fairly responds to a written request from the FDA for such data. The data does not need to show that the product is effective in the pediatric population studied; rather, if the clinical trial is deemed to fairly respond to the FDA's request, additional protection is granted. If reports of requested pediatric studies are submitted to and accepted by FDA within the statutory time limits, whatever statutory or regulatory periods of exclusivity or Orange Book listed patent protection that cover the drug are extended by six months. This is not a patent term extension, but it effectively extends the regulatory period during which the FDA cannot approve an ANDA or 505(b)(2) application owing to regulatory exclusivity or listed patents. When any product is approved, we will evaluate seeking pediatric exclusivity as appropriate.
Orphan Drug Exclusivity. The Orphan Drug Act provides incentives for the development of drugs intended to treat rare diseases or conditions, which generally are diseases or conditions affecting less than 200,000 individuals in the U.S. If a sponsor demonstrates that a drug product qualifies for orphan drug designation, the FDA grants ODD to the product for that use. The benefits of ODD include research and development tax credits and exemption from user fees. A drug that is approved for the orphan drug designated indication generally is granted seven years of orphan drug exclusivity. During that period, the FDA generally may not approve any other application for the same product for the same indication, although there are exceptions, most notably when the later product is shown to be clinically superior to the product with exclusivity. The FDA can revoke a product’s orphan drug exclusivity under certain circumstances, including when the product sponsor is unable to assure the availability of sufficient quantities of the product to meet patient needs. Orphan drug exclusivity does not prevent the FDA from approving a different drug for the same disease or condition, or the same drug for a different disease or condition.
All our clinical-stage product candidates – GTX-104, GTX-102, and GTX-101 – have an ODD.
U.S. Healthcare Reform
The Patient Protection and Affordable Care Act, as amended (the "Affordable Care Act"), is a sweeping measure intended to expand healthcare coverage within the U.S., primarily through the imposition of health insurance mandates on employers and individuals and expansion of the Medicaid program. This law substantially changed the way healthcare is financed by both governmental and private insurers and significantly impacts the pharmaceutical industry. Changes that may affect our business include those governing enrollment in federal healthcare programs, reimbursement changes, benefits for patients within a coverage gap in the Medicare Part D prescription drug program (commonly known as the "donut hole"), rules regarding prescription drug benefits under the health insurance exchanges, changes to the Medicaid Drug Rebate program, expansion of the Public Health Service Act's 340B drug pricing program (340B Program), fraud and abuse, and enforcement. These changes impact existing government healthcare programs and have resulted in the development of new programs, including Medicare payment for performance initiatives.
Some states have elected not to expand their Medicaid programs to individuals with an income of up to 133% of the federal poverty level, as is permitted under the Affordable Care Act. For each state that does not choose to expand its Medicaid program, there may be fewer insured patients overall, which could impact our sales of product candidates for which we receive regulatory approval, and our business and financial condition. Where new patients receive insurance coverage under any of the new Medicaid options made available through the Affordable Care Act, the possibility exists that manufacturers may be required to pay Medicaid rebates on drugs used under these circumstances, a decision that could impact manufacturer revenues.
Certain provisions of the Affordable Care Act have been subject to judicial challenges, as well as efforts to modify them or to alter their interpretation and implementation. For example, on December 22, 2017, the U.S. government signed into law comprehensive tax legislation, referred to as the Tax Cuts and Jobs Act, which includes a provision repealing, effective January 1, 2019, the tax-based shared responsibility payment imposed by the Affordable Care Act on certain individuals who fail to maintain qualifying health coverage for all or part of a year that is commonly referred to as the “individual mandate.” Further, the Bipartisan Budget Act of 2018, among other things, amended the Medicare statute to reduce the coverage gap in most Medicare drug plans, commonly known as the “donut hole,” by raising the required manufacturer point-of-sale discount for pharmaceutical manufacturers who participate in Medicare Part D from 50% to 70% off the negotiated price effective as of January 1, 2019. The Inflation Reduction Act of 2022 replaces the Part D coverage gap discount program with a new Part D manufacturer discount program beginning in 2025.
It is unclear how efforts to modify or invalidate the Affordable Care Act or its implementing regulations, or portions thereof, will affect the Affordable Care Act or our business. Additional legislative changes, regulatory changes, and further judicial challenges related to the Affordable Care Act remain possible. Any such changes could decrease the number of individuals with health coverage. It is possible that the Affordable Care Act, as currently enacted or as it or its implementation may be modified in the future, and other healthcare reform measures that may be adopted in the future, could have a material adverse effect on our industry generally and on our ability to maintain or increase sales of our product candidates for which we receive regulatory approval or to successfully commercialize our product candidates.
25
Additionally, on December 20, 2019, then-President Trump signed the Further Consolidated Appropriations Act for 2020 into law (P.L. 116-94) that includes a piece of bipartisan legislation called the Creating and Restoring Equal Access to Equivalent Samples Act of 2019 or “the CREATES Act.” The CREATES Act aims to address the concern articulated by both the FDA and others in the industry that some brand manufacturers have improperly restricted the distribution of their products, including by invoking the existence of a REMS for certain products, to deny generic product developers access to samples of brand products. Because generic product developers need samples to conduct certain comparative testing required by the FDA, some have attributed the inability to timely obtain samples as a cause of delay in the entry of generic products. To remedy this concern, the CREATES Act establishes a private cause of action that permits a generic product developer to sue the brand manufacturer to compel it to furnish the necessary samples on “commercially reasonable, market-based terms.”
Healthcare Privacy Laws
We may be subject to laws and regulations covering data privacy and the protection of health-related and other personal information. The legislative and regulatory landscape for privacy and data protection continues to evolve, and there has been an increasing focus on privacy and data protection issues which may affect our business. Numerous federal and state laws and regulations, including state security breach notification laws, state health information privacy laws and federal and state consumer protection laws, govern the collection, use, disclosure, and protection of personal information. Failure to comply with such laws and regulations could result in government enforcement actions and create liability for us (including the imposition of significant penalties), private litigation and/or adverse publicity that could negatively affect our business.
Foreign Corrupt Practices Act
In addition, the U.S. Foreign Corrupt Practices Act of 1997 prohibits corporations and their intermediaries from engaging in certain activities to obtain or retain business or to influence a person working in an official capacity. It is illegal to pay, offer to pay or authorize the payment of anything of value to any official of another country, government staff member, political party or political candidate to obtain or retain business or to otherwise influence a person working in that capacity.
Human Capital Resources
As of March 31, 2024, we had a total of four full-time employees and utilized the services of three full-time consultants, all of whom are located in the United States. Our employees have significant prior experience within the pharmaceutical, and biotech industries. We continue to focus on building a high performing organization through our emphasis on accountability for results as measured by our performance development process. To help ensure that employees fully understand the Company’s long-term strategy, and how their work contributes to the Company’s success, we utilize a variety of channels to facilitate open and direct communication, including: regular calls with all employees, and ongoing update communications as needed.
Recent Developments
Announcement of appointment of Robert J. DelAversano as Principal Financial Officer
On January 5, 2024, we announced the appointment of Robert J. DelAversano as Acasti's new Vice President, Finance, in which capacity he serves as our Principal Financial Officer and Principal Accounting Officer, succeeding Brian Ford, our former interim Chief Financial Officer. Mr. Ford continues to serve as a financial consultant on an as-needed basis.
Change in Certifying Accountant
On December 11, 2023, the Audit Committee (the "Audit Committee") of our Board of Directors (the "Board") recommended to the Board and the Board approved the dismissal of Ernst & Young LLP (Canada) ("E&Y") as our independent registered public accounting firm. The report of E&Y on our consolidated financial statements as of and for the fiscal year ended March 31, 2023 did not contain any adverse opinion or a disclaimer of opinion, nor was it qualified or modified as to uncertainty, audit scope or accounting principles. On December 11, 2023, in connection with the dismissal of E&Y, the Audit Committee recommended to the Board and the Board approved the engagement of KPMG LLP (U.S.) ("KPMG") as our new independent registered public accounting firm to audit the Company’s consolidated financial statements for the fiscal year ending March 31, 2024. The decision to engage KPMG was recommended by the Audit Committee, and approved by the Board, after taking into account KPMG’s location in the United States, the results of a competitive review process and other business factors.
Dosing of First Patient
26
On October 23, 2023, we enrolled our first patient in our STRIVE-ON Phase 3 clinical trial. We expect this safety trial to be the final clinical step required to seek FDA approval under the 505(b)(2) regulatory pathway. Patient enrollment in the STRIVE-ON Phase 3 trial is continuing, and potential NDA submission with the FDA is anticipated to occur in the first half of calendar 2025.
September 2023 Private Placement Offering
On September 24, 2023, we entered into a securities purchase agreement (the “Purchase Agreement”) with certain institutional and accredited investors in connection with a private placement offering of our securities (the “Offering”). Pursuant to the Purchase Agreement, we sold 1,951,371 Class A common shares, no par value per share (the “Common Shares”), at a purchase price of $1.848 per Common Share and pre-funded warrants (the “Pre-funded Warrants”) to purchase up to 2,106,853 Common Shares at a purchase price equal to the purchase price per Common Share less $0.0001. Pursuant to the Purchase Agreement, we also issued to such institutional and accredited investors common warrants (the “Common Warrants”) to purchase Common Shares, exercisable for an aggregate of 2,536,391 Common Shares (the “Warrant Shares”). Under the terms of the Purchase Agreement, for each Common Share and each Pre-funded Warrant issued in the Offering, an accompanying five-eighths (0.625) of a Common Warrant was issued to the purchaser thereof.
The Offering closed on September 25, 2023. Shore Pharma LLC, an entity that was controlled by Vimal Kavuru, the Chair of our Board of Directors, at the time of the Offering and SS Pharma LLC, the beneficial owners of 6.9% and 5.5%, respectively, of our Common Shares outstanding prior to the Offering, each a related party of ours, participated in the Offering. The net proceeds to us from the Offering were approximately $7.3 million, after deducting fees and expenses.
Pursuant to the terms of the Purchase Agreement, we agreed to register for resale the Common Shares sold in the Offering and the Warrant Shares. On October 6, 2023, we filed a resale Registration Statement on Form S-3 with the SEC, registering the Common Shares sold in the Offering and the Warrant Shares for resale. The resale Registration Statement on Form S-3 was declared effective on October 16, 2023.
Announcement of compliance with the Nasdaq minimum bid price requirement
On July 24, 2023, we received written notice (the “Notification Letter”) from the Nasdaq Stock Market LLC (“Nasdaq”) notifying us that we had regained compliance with the minimum bid price requirement set forth in Nasdaq Listing Rule 5550(a)(2) for continued listing on Nasdaq. The Notification Letter was sent following the implementation of a 1-for-6 reverse split of our Common Shares (the “Reverse Stock Split”), which became effective on July 10, 2023.
Reverse stock split
On June 29, 2023, our Board approved an amendment to our Articles of Incorporation (the “Articles of Incorporation”) to implement the Reverse Stock Split. On July 4, 2023, we filed Articles of Amendment to our Articles of Incorporation with the Registraire des entreprises du Québec, to implement the Reverse Stock Split.
Announcement of successful submission of pivotal GTX-104 Phase 3 safety study protocol with FDA and implementation of strategic realignment plan
On May 8, 2023, we announced the successful submission to the FDA of GTX-104's full protocol of our pivotal Phase 3 safety trial and implementation of a strategic realignment plan to maximize shareholder value.
Key strategies implemented were:
In connection with the transformation of our operating model, we appointed the following industry experts to our senior management team:
27
Following the realignment, the Company is a smaller, more focused organization, based in the United States, and concentrated on its development of its lead product GTX-104.
Corporate Structure
Acasti was incorporated on February 1, 2002 under Part 1A of the Companies Act (Québec) under the name “9113-0310 Québec Inc.” On February 14, 2011, the Business Corporations Act (Québec) (“QBCA”), came into effect and replaced the Companies Act (Québec). We are now governed by the QBCA. On August 7, 2008, pursuant to a Certificate of Amendment, we changed our name to “Acasti Pharma Inc.” We became a reporting issuer in the Province of Québec on November 17, 2008. On December 18, 2019, we incorporated a new wholly owned subsidiary named Acasti Innovation AG ("AIAG"), under the laws of Switzerland for the purpose of future development of our intellectual property and for global distribution of our products. AIAG currently does not have any operations. On August 27, 2021, Acasti completed its acquisition of Grace Therapeutics via a merger. Following completion of the merger, Grace became a wholly owned subsidiary of Acasti and was renamed Acasti Pharma U.S. Inc.
Available Information
This Annual Report on Form 10-K, our quarterly reports on Form 10-Q, our current reports on Form 8-K, and any amendments to these reports are filed, or will be filed, as applicable, with the SEC, and the Canadian Securities Administrators (“CSA”). These reports are available free of charge on our website, www.acasti.com, as soon as reasonably practicable after we electronically file such reports with or furnish such reports to the SEC and the CSA. Information contained on, or accessible through, our website is not a part of this Annual Report on Form 10-K, and the inclusion of our website address in this document is an inactive textual reference.
Additionally, our filings with the SEC may be accessed through the SEC’s website at www.sec.gov and our filings with the CSA may be accessed through the CSA’s System for Electronic Document Analysis and Retrieval at www.sedar.com.
28
Item 1A. Risk Factors
Risks Factors Relating to our Business
We may not achieve our publicly announced milestones on time, or at all.
From time to time, we may publicly announce the timing of certain events that we expect to occur, such as the anticipated timing of results from our clinical trials and the timing of an upcoming new drug application ("NDA”) filing. These statements are forward-looking and are based on the best estimate of management at the time relating to the occurrence of the events. However, the actual timing of these events may differ from what has been publicly disclosed. The timing of events such as completion of a clinical trial, discovery of a new product candidate, filing of an application to obtain regulatory approval, beginning of commercialization of products, completion of a strategic partnership, or announcement of additional clinical trials for a product candidate may ultimately vary from what is publicly disclosed. These variations in timing may occur as a result of different events, including the nature of the results obtained during a clinical trial or during a research phase, problems with a supplier or a distribution partner or any other event having the effect of delaying the publicly announced timeline. We undertake no obligation to update or revise any forward-looking information, whether as a result of new information, future events or otherwise, except as otherwise required by law. Any variation in the timing of previously announced milestones could have a material adverse effect on our business, financial condition or operating results and the trading price of our common shares.
We are heavily dependent on the success of our lead drug candidate, GTX-104
Our business and future success are substantially dependent on our ability to successfully and timely develop, obtain regulatory approval for, and commercialize our lead product candidate, GTX-104. Any delay or setback in the development of GTX-104 could adversely affect our business. Our planned development, approval and commercialization of GTX-104 may fail to be completed in a timely manner or at all. As part of our recent strategic realignment plan, we determined to focus primarily on the development of GTX-104, which concentrates the level of our drug development risk on one drug candidate. We cannot provide assurance that we will be able to obtain approval for GTX-104 or any other of our drug candidates from the U.S. Food and Drug Administration (the “FDA”) or any foreign regulatory authority or that we will obtain such approval in a timely manner.
We may not be able to maximize value from our de-prioritized drug candidates, GTX-102 and GTX-101, through either development, out-licensing or sale.
Our GTX-102 and GTX-101 drug candidates are at an earlier development stage than GTX-104 and will require additional time and resources to develop. As part of our recent strategic realignment plan, we determined to focus primarily on the development of GTX-104 and to de-emphasize the development of GTX-102 and GTX-101. While we will continue to seek ways to maximize the value of GTX-102 and GTX-101, including through subsequent development, out-licensing or sale, we may not be successful in doing so.
We may not be able to maintain our operations and advance our research and development and commercialization of our GTX-104 lead drug candidate without additional funding.
We have incurred operating losses and negative cash flows from operations since our inception. To date, we have financed our operations through public offerings and private placements of securities, proceeds from exercises of warrants, rights and options, and receipt of research tax credits and research grant programs. Our cash and cash equivalents and short-term investments were $23.0 million as of March 31, 2024 and $27.9 million as of March 31, 2023.
Our current assets, as of March 31, 2024, are projected to support our current liabilities as at that date when combined with the projected level of our expenses for the next twelve months, including fully funding the completion of our Phase 3 program for GTX-104. We expect that additional time and capital will be required by us to file an NDA to obtain FDA approval for GTX-104 in the United States, to further scale up our manufacturing capabilities, and to complete marketing and other pre-commercialization activities. Consequently, we expect our existing cash and cash equivalents will be sufficient to fund our operations into the second calendar quarter of 2026. Based on the steps we are taking in our strategic realignment plan to focus primarily on the development of GTX-104 and to de-emphasize the development of GTX-102 and GTX-101, we believe that our existing cash and cash equivalents will enable us to fund our operating expenses and capital expenditure requirements beyond the completion of our Phase 3 trials for GTX-104. To fully execute our business plan, we plan to raise the necessary capital primarily through additional securities offerings and multiple sources of non-dilutive capital, such as grants or loans and strategic alliances. If we are unable to raise additional capital in sufficient amounts or on terms acceptable to us, we may have to significantly delay the research and development and commercial launch of our GTX-104. If we determine to continue development of GTX-102 and GTX-101, significant additional funding will be needed. Unexpected negative results in our clinical programs for our lead drug candidate may affect our ability to raise additional capital and/or complete strategic development and/or distribution partnerships to support the commercial launch of our lead drug candidate. Additional funding from
29
third parties may not be available on acceptable terms or at all to enable us to continue with the research and development and commercialization of our lead drug candidate.
Business disruptions could seriously harm our future revenue and financial condition and increase our costs and expenses.
Our operations, and those of our suppliers, third-party manufacturers and other contractors and consultants could be subject to earthquakes, power shortages, telecommunications failures, water shortages, floods, hurricanes, typhoons, fires, extreme weather conditions, medical pandemics and other natural or man-made disasters or business interruptions, for which we are predominantly self-insured. The occurrence of any of these business disruptions could seriously harm our operations and financial condition and increase our costs and expenses. We rely on third-party manufacturers to manufacture our drug candidate products. Our ability to obtain supplies of drug candidate products could be disrupted if the operations of our manufacturers and suppliers are affected by a man-made or natural disaster or other business interruption.
Our future success depends on our ability to retain key executives and to attract, retain and motivate qualified personnel.
We are highly dependent on the principal members of our executive team. While members of our executive team have significant industry experience, they have not been with the Company for long. Any of our executive officers could leave our employment at any time, as all of our employees are “at will” employees. Also, as part of our strategic realignment, we significantly reduced the number of our employees while we shift the base of our operations from Canada to the United States. As a result, in the process of shifting the base of our operations to the United States, we will have to recruit employees from the industry employment market in the United States. Recruiting and retaining qualified employees for our business, including scientific and technical personnel, will also be critical to our success. There is currently a shortage of skilled executives and other personnel in our industry, which is likely to continue. As a result, competition for skilled personnel is intense and the turnover rate can be high. As we rebuild our organization in accordance with our strategic realignment, we may not be able to attract and retain personnel on acceptable terms given the competition among numerous pharmaceutical companies for individuals with similar skill sets. In addition, failure to succeed in clinical studies may make it more challenging to recruit and retain qualified personnel. The inability to recruit key executives or the loss of the services of any executive or key employee might impede the progress of our development and commercialization objectives.
We may need to expand our organization, and we may experience difficulties in managing this growth, which could disrupt our operations and our ability to compete.
If our drug development efforts are successful, we expect to expand our employee base to increase our managerial, scientific, engineering, operational, sales, marketing, financial and other resources and to hire more consultants and contractors. Future growth would impose significant additional responsibilities on our management, including the need to identify, recruit, maintain, motivate, and integrate additional employees, consultants and contractors. We may not be able to effectively manage the expansion of our operations, which may result in weaknesses in our infrastructure, give rise to operational mistakes, loss of business opportunities, loss of employees and reduced productivity among remaining employees. Future growth could require significant capital expenditures and may divert financial resources from other projects, such as the development of our existing or future product candidates. Our future financial performance and our ability to sell and commercialize our product candidates, if approved, and compete effectively will depend, in part, on our ability to effectively manage any future growth.
We face potential product liability, and if claims are brought against us, we may incur substantial liability.
The use of our product candidates in clinical trials, and the sale of any drug candidates for which we obtain marketing approval, exposes us to the risk of product liability claims. Product liability claims might be brought against us by consumers, healthcare providers, pharmaceutical companies or others selling or otherwise coming into contact with our product candidates. If we cannot successfully defend against product liability claims, we could incur substantial liability and costs. In addition, regardless of merit or eventual outcome, product liability claims may result in:
30
Our current product liability insurance coverage may not be sufficient to reimburse us for any expenses or losses we may suffer. Moreover, insurance coverage is becoming increasingly expensive, and, in the future, we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses due to liability. A successful product liability claim or series of claims brought against us could cause our share price to decline and, if judgments exceed our insurance coverage, could adversely affect our results of operations and business.
We rely significantly on information technology and any failure, inadequacy, interruption, or security lapse of that technology, including any cybersecurity incidents, could harm our ability to operate our business effectively.
Despite the implementation of security measures, our internal computer systems, and those of third parties with which we contract are vulnerable to damage from cyber-attacks, computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. System failures, accidents or security breaches could cause interruptions in our operations and could result in a material disruption of our drug product development and clinical activities and business operations, in addition to possibly requiring substantial expenditures of resources to remedy. The loss of drug product development or clinical trial data could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and our development programs, and the development of our product candidates could be delayed.
Risks Related to Development, Testing and Commercialization of Our Products
Even if our drug candidates receive regulatory approval in the United States, we may never obtain regulatory approval or successfully commercialize our products outside of the United States.
Our business plan is highly dependent upon our ability to obtain regulatory approval to market and commercialize our lead drug candidate, GTX-104 in the United States. As GTX-104 is currently the focus of our drug development program, the failure to commercialize it would have a material adverse effect on our ability to execute on our business plan and generate revenue. In addition, even if we obtain U.S. regulatory approvals to commercialize GTX-104, we may not be able to do so in other international jurisdictions.
We are subject to uncertainty relating to healthcare reform measures and reimbursement policies which, if not favorable to our drug candidates, could hinder or prevent our drug candidates’ commercial success.
Our ability to commercialize our drug candidates successfully will depend in part on the extent to which governmental authorities, private health insurers and other third-party payors establish appropriate coverage and reimbursement levels for our drug candidates and related treatments. As a threshold for coverage and reimbursement, third-party payors generally require that drug products have been approved for marketing by the FDA. Third-party payors are increasingly imposing additional requirements and restrictions on coverage and limiting reimbursement levels for medical products. These restrictions and limitations influence the purchase of healthcare services and products. The cost containment measures that healthcare payors and providers are instituting and the effect of any healthcare reform could significantly reduce our revenues from the sale of any approved drug. We cannot provide any assurances that we will be able to obtain third-party coverage or reimbursement for our drug candidates in whole or in part.
In the United States, there have been a number of legislative and regulatory changes to the healthcare system in ways that could affect our future revenues and profitability and the future revenues and profitability of our potential customers. Under the prescription drug benefit, Medicare beneficiaries can obtain prescription drug coverage from private sector plans that are permitted to limit the number of prescription drugs that are covered in each therapeutic category and class on their formularies. If our products are not widely included on the formularies of these plans, our ability to market our products to the Medicare population could be harmed.
There also have been, and likely will continue to be, legislative and regulatory proposals at the federal and state levels directed at containing or lowering the cost of healthcare. We cannot predict the initiatives that may be adopted in the future. The continuing efforts of the government, insurance companies, managed care organizations and other payors of healthcare costs to contain or reduce costs of healthcare may adversely affect one or more of the following:
31
Any of these scenarios could harm our ability to market our products and generate revenues. It is also possible that other proposals having a similar effect will be adopted.
Our commercial success depends upon attaining significant market acceptance of our drug candidates and drug products, if approved, among physicians, nurses, pharmacists, patients and the medical community.
Even if we obtain regulatory approval for our drug product candidates, our drug product candidates may not gain market acceptance among physicians, nurses, pharmacists, patients, the medical community or third-party payors, which is critical to commercial success. Market acceptance of our drug candidates and any drug product for which we receive approval depends on a number of factors, including:
If our drug candidates or drug products, if approved, fail to achieve an adequate level of acceptance by physicians, nurses, pharmacists, patients, and the medical community, we will be unable to generate significant revenues, and we may not become or remain profitable.
If we are unable to establish sales and marketing capabilities or enter into agreements with third parties to market and sell our drug candidates, we may be unable to generate any revenue.
Although we intend to establish a small, focused, specialty sales and marketing organization to promote GTX-104, if approved for marketing in the United States, we currently have no such organization and the cost of establishing and maintaining such an organization may exceed the benefit of doing so. We believe that GTX-102 could also be marketed by a small, focused, specialty sales and marketing organization if and when we decide to resume development of GTX-102. Given the size of its potential market, we anticipate that commercializing GTX-101 would require entering into a strategic partnership with a larger marketing partner, if GTX-101 is approved by the FDA for marketing, and the ability to find any such strategic partnership would be uncertain. If we are unable to establish adequate sales, marketing and distribution capabilities, whether independently or with third parties, we may not be able to generate sufficient product revenue and may not become profitable. We will be competing with many companies that currently have extensive and well-funded marketing and sales operations. Without an internal team or the support of a third-party to perform marketing and sales functions, we may be unable to compete successfully against these more established companies.
If we obtain approval to commercialize any approved drug products outside of the United States, a variety of risks associated with international operations could materially adversely affect our business.
If any of our drug candidates are approved for commercialization, we may enter into agreements with third parties to market these drug products outside the United States. We expect that we will be subject to additional risks related to entering into international business relationships, including:
32
If we are unable to differentiate our drug candidates from branded reference drugs or existing generic therapies for similar treatments, or if the FDA or other applicable regulatory authorities approve generic products that compete with any of our drug candidates, our ability to successfully commercialize our drug candidates would be adversely affected.
Although we believe that our drug candidates will be clinically differentiated from branded reference drugs and their generic counterparts, if any, it is possible that such differentiation will not impact our market position. If we are unable to achieve significant differentiation for our product candidates against other drugs, the opportunity for our product candidates to achieve premium pricing and be commercialized successfully would be adversely affected.
In addition to existing branded reference drugs and the related generic products, the FDA or other applicable regulatory authorities may approve generic products that compete directly with our drug candidates, if approved. Once an NDA, including a 505(b)(2) application, is approved, the product covered thereby becomes a “listed drug” which can, in turn, be cited by potential competitors in support of approval of an abbreviated new drug application (“ANDA”). The Federal Food, Drug, and Cosmetic Act (“FDCA”), FDA regulations and other applicable regulations and policies provide incentives to manufacturers to create modified, non-infringing versions of a drug to facilitate the approval of an ANDA for generic substitutes. These manufacturers might only be required to conduct a relatively inexpensive study to show that their product has the same active ingredient(s), dosage form, strength, route of administration and conditions of use or labeling as our product candidate and that the generic product is bioequivalent to ours, meaning it is absorbed in the body at the same rate and to the same extent as our drug product. These generic equivalents, which must meet the same quality standards as branded pharmaceuticals, would be significantly less costly than ours to bring to market and companies that produce generic equivalents are generally able to offer their drug products at lower prices. After the introduction of a generic competitor, a significant percentage of the sales of any branded drug product is typically lost to the generic drug product. Accordingly, competition from generic equivalents of our drug candidates would materially adversely impact our ability to successfully commercialize our drug candidates.
We face significant competition from other biotechnology and pharmaceutical companies, and our operating results will suffer if we fail to compete effectively.
The biopharmaceutical industry is intensely competitive and subject to rapid and significant technological change. We expect to have competitors both in the United States and internationally, including major multinational pharmaceutical companies, biotechnology companies and universities and other research institutions.
Many of our competitors have substantially greater financial, technical and other resources, such as larger research and development staff and experienced marketing and manufacturing organizations. If our competitors market products that are more effective, safer or less expensive than our drug products, if any, or that reach the market sooner than our drug products, if any, we may enter the market too late in the cycle and may not achieve commercial success. In addition, the biopharmaceutical industry is characterized by rapid technological change. If we fail to stay at the forefront of technological change, we may be unable to compete effectively. Technological advances or drug products developed by our competitors may render our drug products, if any, or drug candidates obsolete, less competitive or not economical.
We could incur substantial costs and disruption to our business and delays in the launch of our drug candidates if our competitors and/or collaborators bring legal actions against us, which could harm our business and operating results.
We cannot predict whether our competitors or potential competitors, may bring legal actions against us based on our research, development, and commercialization activities, as well as any drug candidates or drug products resulting from these activities, claiming, among other things, infringement of their intellectual property rights, breach of contract or other legal theories. If we are forced to defend any such lawsuits, whether they are with or without merit or are ultimately determined in our favor, we may face costly litigation and diversion of technical and management personnel. These lawsuits could hinder our ability to enter the market early with our drug candidates and thereby hinder our ability to influence usage patterns when fewer, if any, of our potential competitors have entered such
33
market, which could adversely impact our potential revenue from such drug candidates. Some of our competitors have substantially greater resources than we do and could be able to sustain the cost of litigation to a greater extent and for longer periods of time than we could. Furthermore, an adverse outcome of a dispute may require us: to pay damages, potentially including treble damages and attorneys’ fees, if we are found to have willfully infringed a party’s patent or other intellectual property rights; to cease making, licensing or using products that are alleged to incorporate or make use of the intellectual property of others; to expend additional development resources to reformulate our products or prevent us from marketing a certain drug; and to enter into potentially unfavorable royalty or license agreements in order to obtain the rights to use necessary technologies. Royalty or licensing agreements, if required, may be unavailable on terms acceptable to us, or at all.
We are subject to numerous complex regulatory requirements and failure to comply with these regulations, or the cost of compliance with these regulations, may harm our business.
The research, testing, development, manufacturing, quality control, approval, labeling, packaging, storage, record-keeping, promotion, advertising, marketing, distribution, possession and use of our drug candidates, among other things, are subject to regulation by numerous governmental authorities in the United States and elsewhere. The FDA regulates drugs under the FDCA, and implementing regulations. Non-compliance with any applicable regulatory requirements can result in refusal of the governmental authority to approve products for marketing, criminal prosecution and fines, warning letters, product recalls or seizure of products, total or partial suspension of production, prohibitions or limitations on the commercial sale of products or refusal to allow the entering into of federal and state supply contracts. FDA and comparable governmental authorities have the authority to withdraw product approvals that have been previously granted. In addition, the regulatory requirements relating to our drug candidates and drug products, if any, may change from time to time and it is impossible to predict what the impact of any such changes may be.
If the FDA does not conclude that our drug candidates satisfy the requirements for the 505(b)(2) regulatory approval pathway, or if the requirements for approval of any of our drug candidates under Section 505(b)(2) are not as we expect, the approval pathway for our drug candidates will likely take significantly longer, cost significantly more and encounter significantly greater complications and risks than anticipated, and in any case may not be successful.
We intend to seek FDA approval through the 505(b)(2) regulatory pathway for our lead drug candidate GTX-104. The Drug Price Competition and Patent Term Restoration Act of 1984, also known as the Hatch-Waxman Act, added Section 505(b)(2) to the FDCA. Section 505(b)(2) permits the filing of an NDA where at least some of the information required for approval comes from studies that were not conducted by or for the applicant.
If the FDA does not allow us to pursue the 505(b)(2) regulatory pathway for GTX-104, we may need to conduct additional clinical trials, provide additional data and information and meet additional standards for regulatory approval. If this were to occur, the time and financial resources required to obtain FDA approval for our drug candidates would likely substantially increase. Moreover, an inability to pursue the 505(b)(2) regulatory pathway could result in new competitive products reaching the market faster than our drug candidates, which could materially adversely impact our competitive position and prospects. Even if we are allowed to pursue the 505(b)(2) regulatory pathway for a drug candidate, we cannot assure you that we will receive the requisite or timely approvals for commercialization of such drug candidate.
In addition, it is possible that our competitors may file citizens’ petitions with the FDA in an attempt to persuade the FDA that our drug candidates, or the clinical studies that support their approval, contain deficiencies. Such actions by our competitors could delay or even prevent the FDA from approving any NDA that we submit under Section 505(b)(2).
Clinical development is a lengthy and expensive process with an uncertain outcome, and results of earlier studies and trials may not be predictive of future trial results. Failure can occur at any stage of clinical development.
Clinical testing, even when utilizing the 505(b)(2) pathway, is expensive and can take many years to complete, and its outcome is inherently uncertain. Failure can occur at any time during the clinical trial process, even with active ingredients that have previously been approved by the FDA as safe and effective. The results of pre-clinical studies and early clinical trials of our drug candidates may not be predictive of the results of later stage clinical trials. A number of companies in the biopharmaceutical industry have suffered significant setbacks in advanced clinical trials due to lack of efficacy or adverse safety profiles, notwithstanding promising results in earlier trials.
Our drug candidates are in various stages of development. Clinical trial failures may occur at any stage and may result from a multitude of factors both within and outside our control, including flaws in formulation, adverse safety or efficacy profile and flaws in trial design, among others. If the trials result in negative or inconclusive results, we or our collaborators may decide, or regulators may require us, to discontinue trials of our drug candidates or conduct additional clinical trials or pre-clinical studies. In addition, data obtained from trials and studies are susceptible to varying interpretations, and regulators may not interpret our data as favorably as we do, which may delay, limit or prevent regulatory approval. For these reasons, our future clinical trials may not be successful.
34
Delays in clinical trials are common and have many causes, and any delay could result in increased costs to us and could jeopardize or delay our ability to obtain regulatory approval and commence drug product sales. We may also find it difficult to enroll patients in our clinical trials, which could delay or prevent development of our drug candidates.
We may experience delays in clinical trials of our drug candidates. Our planned clinical trials may not begin on time, have an effective design, enroll a sufficient number of patients or be completed on schedule, if at all. Our clinical trials can be delayed for a variety of reasons, including:
If initiation or completion of our planned clinical trials is delayed for any of the above reasons or other reasons, our development costs may increase, our regulatory approval process could be delayed and our ability to commercialize and commence sales of our drug candidates could be materially harmed, which could have a material adverse effect on our business.
In addition, identifying and qualifying patients to participate in clinical trials of our drug candidates is critical to our success. The timing of our clinical trials depends on the speed at which we can recruit patients to participate in testing our drug candidates as well as completion of required follow-up periods. We may not be able to identify, recruit and enroll a sufficient number of patients, or those with required or desired characteristics or to complete our clinical trials in a timely manner. Patient enrollment is and completion of the trials are affected by a variety of factors, including:
35
The regulatory approval processes of the FDA and comparable foreign authorities are lengthy, time consuming and inherently unpredictable, and if we are ultimately unable to obtain regulatory approval for our drug candidates, our business will be substantially harmed.
The time required to obtain approval by the FDA and comparable foreign authorities is unpredictable but typically takes many years following the commencement of clinical trials and depends upon numerous factors, including the substantial discretion of the regulatory authorities. In addition, approval policies, regulations or the type and amount of clinical data necessary to gain approval may change during the course of a drug candidate’s clinical development and may vary among jurisdictions. It is possible that none of our existing drug candidates or any drug candidates we may seek to develop will ever obtain regulatory approval in the United States or other jurisdictions.
Our drug candidates could fail to receive regulatory approval for many reasons, including the following:
This lengthy approval process as well as the unpredictability of future clinical trial results may result in us failing to obtain regulatory approval to market our drug candidates, which would harm our business, results of operations and prospects significantly.
We have limited experience using the 505(b)(2) regulatory pathway to submit an NDA or any similar drug approval filing to the FDA, and we cannot be certain that any of our drug candidates will receive regulatory approval. If we do not receive regulatory approvals for our drug candidates, we may not be able to continue our operations. Even if we successfully obtain regulatory approvals to market one or more of our drug candidates, our revenue will be dependent, to a significant extent, upon the size of the markets in the territories for which we gain regulatory approval. If the markets for patients or indications that we are targeting are not as significant as we estimate, we may not generate significant revenue from sales of such drug products, if approved.
Our drug development strategy relies heavily upon the 505(b)(2) regulatory pathway, which requires us to certify that we do not infringe upon third-party patents covering approved drugs. Such certifications often result in third-party claims of intellectual property infringement, the defense of which will be costly and time consuming, and an unfavorable outcome in any litigation may prevent or delay our development and commercialization efforts which would harm our business.
Litigation or other proceedings to enforce or defend intellectual property rights are often complex in nature, may be very expensive and time-consuming, may divert our management’s attention from other aspects of our business and may result in unfavorable outcomes that could adversely impact our ability to launch and market our drug candidates, or to prevent third parties from competing with our drug products and drug candidates.
In particular, our commercial success depends in large part on our avoiding infringement of the patents and proprietary rights of third parties for existing approved drug products. Because we intend to utilize the 505(b)(2) regulatory pathway for the approval of our drug products and drug candidates, we rely in whole or in part on studies conducted by third parties related to those approved drug products.
Because patent applications can take many years to issue, there may be currently pending or subsequently filed patent applications which may later result in issued patents that may be infringed by our drug products or drug candidates. If any third-party patents were held by a court of competent jurisdiction to cover aspects of our drug candidates, including the formulation, method of use, any method or process involved in the manufacture of any of our drug candidates, any molecules or intermediates formed during such manufacturing process or any other attribute of the final product itself, the holders of any such patents may be able to block our ability to commercialize our drug candidates unless we obtain a license under the applicable patents, or until such patents expire. In either case, such a license may not be available on commercially reasonable terms or at all.
36
Parties making claims against us may request and/or obtain injunctive or other equitable relief, which could effectively block our ability to further develop and commercialize one or more of our drug candidates on a temporary or permanent basis. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of employee resources from our business. In the event of a successful claim of infringement against us, we may have to pay substantial damages, including treble damages and attorneys’ fees for willful infringement, obtain one or more licenses from third parties, pay royalties or redesign our infringing products or manufacturing processes, which may be impossible or require substantial time and monetary expenditure. We cannot predict whether any such license would be available at all or whether it would be available on commercially reasonable terms. Furthermore, even in the absence of litigation, we may need to obtain licenses from third parties to advance our research, manufacture clinical trial supplies or allow commercialization of our drug candidates. We may fail to obtain any of these licenses at a reasonable cost or on reasonable terms, if at all. In that event, we would be unable to further develop and commercialize one or more of our drug candidates, which could harm our business significantly. We cannot provide any assurances that third-party patents do not exist which might be enforced against our products, resulting in either an injunction prohibiting our sales, or, with respect to our sales, an obligation on our part to pay royalties and/or other forms of compensation to third parties.
Our employees, independent contractors, principal investigators, consultants, commercial partners and vendors may engage in misconduct or other improper activities, including non-compliance with regulatory standards and requirements.
We are exposed to the risk that our employees, independent contractors, principal investigators, consultants, commercial partners, and vendors may engage in fraudulent conduct or other illegal activity. Misconduct by these parties could include intentional, reckless and/or negligent conduct that violates (1) the laws of the FDA and similar foreign regulatory bodies, including those laws requiring the reporting of true, complete, and accurate information to such regulatory bodies; (2) healthcare fraud and abuse laws of the United States and similar foreign fraudulent misconduct laws; and (3) laws requiring the reporting of financial information or data accurately. Specifically, the promotion, sales and marketing of health care items and services, as well as certain business arrangements in the healthcare industry are subject to extensive laws designed to prevent misconduct, including fraud, kickbacks, self-dealing and other abusive practices. These laws may restrict or prohibit a wide range of pricing, discounting, marketing, structuring and commission(s), certain customer incentive programs and other business arrangements generally. Activities subject to these laws also involve the improper use of information obtained in the course of patient recruitment for clinical trials. It is not always possible to identify and deter employee and other third-party misconduct. The precautions we take to detect and prevent inappropriate conduct may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with these laws. If any such actions are instituted against us, and we are not successful in defending ourselves, those actions could have a significant impact on our business, including the imposition of civil, criminal and administrative penalties, damages, monetary fines, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, contractual damages, reputational harm, diminished profits and future earnings, and curtailment of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
We are required to obtain regulatory approval for each of our drug candidates in each jurisdiction in which we intend to market such products, and the inability to obtain such approvals would limit our ability to realize their full market potential.
In order to market drug products outside of the United States, we must comply with numerous and varying regulatory requirements of other countries regarding safety and efficacy. Clinical trials conducted in one country may not be accepted by regulatory authorities in other countries, and regulatory approval in one country does not mean that regulatory approval will be obtained in any other country. However, the failure to obtain regulatory approval in one jurisdiction may adversely impact our ability to obtain regulatory approval in another jurisdiction. Approval processes vary among countries and can involve additional product testing and validation and additional administrative review periods. Seeking foreign regulatory approval could result in difficulties and costs for us and require additional non-clinical studies or clinical trials which could be costly and time consuming. Regulatory requirements can vary widely from country to country and could delay or prevent the introduction of our drug products in those countries. If we fail to comply with regulatory requirements in international markets or to obtain and maintain required approvals, or if regulatory approval in international markets is delayed, our target market will be reduced and our ability to realize the full market potential of our drug products will be harmed.
Risks Relating to Our Intellectual Property
If we are sued for infringing intellectual property rights of third parties, it will be costly and time consuming, and an unfavorable outcome in that litigation would have a material adverse effect on our business.
Our commercial success also depends upon our ability and the ability of our future collaborators to develop, manufacture, market and sell our drug candidates and to use our proprietary technologies without infringing the proprietary rights of third parties. Numerous U.S. and foreign issued patents and pending patent applications, which are owned by third parties, exist in the fields in which we are developing drug candidates. Because patent applications can take many years to issue, there may be currently pending applications,
37
which may later result in issued patents that our product candidates or proprietary technologies may infringe. Similarly, there may be issued patents relevant to our drug candidates of which we are not aware.
There is a substantial amount of litigation involving patent and other intellectual property rights in the biotechnology and biopharmaceutical industries generally. In particular, the generic drug industry is characterized by frequent litigation between generic drug companies and branded drug companies. If a third-party claims that we infringe its intellectual property rights, we may face a number of issues, including, but not limited to:
We have not conducted an extensive search of patents issued to third parties, and no assurance can be given that third-party patents containing claims covering our drug candidates, technology or methods do not exist, have not been filed, or could not be filed or issued. Because of the number of patents issued and patent applications filed in our technical areas or fields, we believe there is a significant risk that third parties may allege they have patent rights encompassing our products, technology, or methods. Other drug candidates that we may in-license or acquire could be subject to similar risks and uncertainties.
We may be subject to claims that our employees, consultants, or independent contractors have wrongfully used or disclosed alleged confidential information or trade secrets of their other clients or former employers to us.
As is common in the biotechnology and pharmaceutical industry, certain of our employees were formerly employed by other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Moreover, we engage the services of consultants to assist us in the development of our drug candidates, many of whom were previously employed at or may have previously been or are currently providing consulting services to, other biotechnology or pharmaceutical companies, including our competitors or potential competitors. We may be subject to claims that these employees and consultants or we have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers or their former or current customers. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management. Any such litigation would be protracted, expensive, and potentially subject to an unfavorable outcome.
Our success depends in part upon our ability to protect our intellectual property for our branded products and drug candidates.
Our commercial success with respect to our drug products and drug candidates, depends on obtaining and maintaining patent protection in the United States and in other countries and trade secret protection for our drug candidates, proprietary technologies and their uses. Our ability to protect our drug products from unauthorized or infringing use by third parties depends in substantial part on our ability to obtain and maintain valid and enforceable patents.
Due to evolving legal standards relating to patentability, validity and enforceability of patents covering pharmaceutical inventions and the scope of claims made under these patents, our ability to maintain, obtain and enforce patents is uncertain and involves complex legal and factual questions. The patent positions of pharmaceutical and biotechnology companies can be highly uncertain and involve complex legal and factual questions for which important legal principles remain unresolved. Changes in either the patent laws or in the interpretations of patent laws in the United States and other countries may diminish the value and the scope of our intellectual property. Accordingly, we cannot predict the breadth of claims that may be allowed or enforced in our patents or in third-party patents.
The degree of future protection for our proprietary rights is uncertain. Only limited protection may be available and may not adequately protect our rights or permit us to gain or keep our competitive advantage. For example:
38
Proprietary trade secrets and unpatented know-how are also very important to our business. Although we have taken steps to protect our trade secrets and unpatented know-how, including entering into confidentiality agreements with third parties, and confidential information and inventions agreements with certain of our employees, consultants, and advisors, third parties may still obtain this information, or we may be unable to protect our rights. Enforcing a claim that a third-party illegally obtained and is using our trade secrets or unpatented know-how is expensive and time consuming, and the outcome is unpredictable. In addition, courts outside the United States may be less willing to protect trade secret information. Moreover, our competitors may independently develop equivalent knowledge, methods, and know-how, and we would not be able to prevent their use.
If we fail to comply with our obligations in the agreements under which we license rights to technology from third parties, or if the license agreements are terminated for other reasons, we could lose license rights that are important to our business.
We may be a party to a number of technology licenses that are important to our business and expect to enter into additional licenses in the future. Our existing license agreements impose, and we expect that future license agreements will impose, on us, various development, regulatory and/or commercial diligence obligations, payment of milestones and/or royalties and other obligations. Under these agreements, we must rely on our licensor to comply with their obligations under the primary license agreements under which such third-party obtained rights in the applicable intellectual property, where we may have no relationship with the original licensor of such rights. If our licensors fail to comply with their obligations under these upstream license agreements, the original third-party licensor may have the right to terminate the original license, which may terminate our sublicense. If this were to occur, we would no longer have rights to the applicable intellectual property unless we are able to secure our own direct license with the owner of the relevant rights, which we may not be able to do at a reasonable cost or on reasonable terms, which may impact our ability to continue to develop and commercialize our drug candidates and companion diagnostic incorporating the relevant intellectual property. If we fail to comply with our obligations under our license agreements, or we are subject to a bankruptcy or insolvency, the licensor may have the right to terminate the license. In the event that any of our important technology licenses were to be terminated by the licensor, we would likely cease further development of the related program or be required to spend significant time and resources to modify the program to not use the rights under the terminated license.
We may be subject to claims challenging the inventorship or ownership of our patents and other intellectual property.
We may also be subject to claims that former employees, collaborators or other third parties have an ownership interest in our patents or other intellectual property. We may be subject to ownership disputes in the future arising, for example, from conflicting obligations of consultants or others who are involved in developing our drug candidates and companion diagnostic. Litigation may be necessary to defend against these and other claims challenging inventorship or ownership. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, valuable intellectual property. Such an outcome could have a material adverse effect on our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
Intellectual property rights do not necessarily address all potential threats to our competitive advantage.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations and may not adequately protect our business or permit us to maintain our competitive advantage. The following examples are illustrative:
39
Should any of these events occur, they could significantly harm our business, results of operations and prospects.
We may not be able to protect our intellectual property rights throughout the world.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of some countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets and other intellectual property protection, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate, and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
If our estimates or judgments relating to our critical accounting policies for intangible assets prove to be incorrect, further impairment charges could result.
We carry a significant amount of intangible assets on our consolidated balance sheet, associated with acquired in process research and development. In the ordinary course of business, circumstances may arise, including manifestation of any of the risks identified in this section, that could result in further recognition that the carrying values of our assets may not be recovered from future operations. Under such circumstances, it is possible we may be required to further impair our asset values to the extent that their remaining value after any such impairment can be recovered by our business going forward. Intangible assets with an indefinite useful life are subject to an impairment review at least annually.
Risks Related to Our Dependence on Third Parties
Our contract manufacturers may encounter manufacturing failures that could delay the clinical development or regulatory approval of our drug candidates, or their commercial production, if approved.
Any performance failure on the part of any of our manufacturers could delay the clinical development or regulatory approval of our drug candidates. Our manufacturers may encounter difficulties involving, among other things, production yields, regulatory compliance, quality control and quality assurance, as well as shortages of qualified personnel. Approval of our drug candidates could be delayed, limited, or denied if the FDA does not approve and maintain the approval of our contract manufacturer’s processes or facilities. Moreover, our contract manufacturers may encounter difficulties that have a negative impact on our operations and business. Our manufacturers may encounter difficulties with the manufacturing processes required to manufacture commercial quantities of our drug candidates or the quantities needed for our pre-clinical studies or clinical trials. Such difficulties could result in delays in our pre-clinical studies, clinical trials, and regulatory submissions, in the commercialization of our drug candidates. Further, development of large-scale manufacturing processes may require additional validation studies, which the FDA must review and approve. If any of our manufacturers fail to deliver the required commercial quantities or quantities needed for our pre-clinical studies and clinical trials on a timely basis and upon terms that we find acceptable, we may be unable to meet demand for any of our drug candidates that are approved and could lose potential revenue.
Certain changes in the manufacturing process or procedure, including a change in the location where the drug candidate is manufactured or a change of a third-party manufacturer, generally require prior FDA, or foreign regulatory authority, review and/or approval of the manufacturing process and procedures in accordance with current Good Manufacturing Practice (“cGMP”). We may need to conduct
40
additional pre-clinical studies and clinical trials to support approval of such changes. This review may be costly and time-consuming and could delay or prevent the launch of a drug candidate.
We rely on third parties to conduct our pre-clinical studies and clinical trials. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, we may not be able to obtain regulatory approval for or commercialize our drug candidates and our business could be substantially harmed.
We have relied upon and plan to continue to rely upon third-party CROs to monitor and manage data for our pre-clinical and clinical programs. We rely on these parties for execution of our pre-clinical studies and clinical trials, and control only certain aspects of their activities. Nevertheless, we are responsible for ensuring that each of our trials is conducted in accordance with the applicable protocol, legal, regulatory, and scientific standards and our reliance on the CROs does not relieve us of our regulatory responsibilities. We and our CROs are required to comply with FDA laws and regulations regarding current good clinical practice (“GCP”), which are also required by the Competent Authorities of the Member States of the European Economic Area and comparable foreign regulatory authorities in the form of International Conference on Harmonization, guidelines for all of our drug candidates in clinical development. Regulatory authorities enforce GCP through periodic inspections of trial sponsors, principal investigators, and trial sites. If we or any of our CROs fail to comply with applicable GCP, the clinical data generated in our clinical trials may be deemed unreliable and the FDA or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving our marketing applications. We cannot assure you that upon inspection by a given regulatory authority, such regulatory authority will determine that any of our clinical trials comply with GCP regulations. In addition, our clinical trials must be conducted with product produced under cGMP regulations. While we have agreements governing activities of our CROs, we have limited influence over their actual performance. In addition, portions of the clinical trials for our drug candidates are expected to be conducted outside of the United States, which will make it more difficult for us to monitor CROs and perform visits of our clinical trial sites and will force us to rely heavily on CROs to ensure the proper and timely conduct of our clinical trials and compliance with applicable regulations, including GCP. Failure to comply with applicable regulations in the conduct of the clinical trials for our drug candidates may require us to repeat clinical trials, which would delay the regulatory approval process.
We rely on third parties to manufacture commercial and clinical supplies of our drug candidates, and we intend to rely on third parties to manufacture commercial supplies of any approved drug products. The commercialization of any of our drug products could be stopped, delayed, or made less profitable if those third parties fail to provide us with sufficient quantities of active pharmaceutical ingredients, excipients, or drug products, or fail to do so at acceptable quality levels or prices or fail to maintain or achieve satisfactory regulatory compliance.
We do not own any manufacturing facilities, and we do not currently, and do not expect in the future, to independently conduct any aspects of our product manufacturing and testing, or other activities related to the clinical development and commercialization of our drug candidates. We currently rely, and expect to continue to rely, on third parties with respect to these items, and control only certain aspects of their activities.
Any of these third parties may terminate their engagements with us at any time. If we need to enter into alternative arrangements, it could delay our drug candidate development and commercialization activities. Our reliance on these third parties reduces our control over these activities but does not relieve us of our responsibility to ensure compliance with all required legal, regulatory, and scientific standards and any applicable trial protocols. If these third parties do not successfully carry out their contractual duties, meet expected deadlines or conduct our studies in accordance with regulatory requirements or our stated study plans and protocols, we will not be able to complete, or may be delayed in completing, clinical trials required to support future regulatory submissions and approval of our drug candidates.
More generally, manufacturers of pharmaceutical products often encounter difficulties in production, particularly in scaling up and validating initial production. These problems include difficulties with production costs and yields, quality control, including stability of the product, quality assurance testing, shortages of qualified personnel, as well as compliance with strictly enforced federal, state, and foreign regulations. Additionally, our manufacturers may experience manufacturing difficulties due to resource constraints or as a result of labor disputes or unstable political environments. If our manufacturers were to encounter any of these difficulties, or otherwise fail to comply with their contractual obligations, our ability to make product candidates available for clinical trials and development purposes or to commercialize any of our product candidates in the United States would be jeopardized. Any delay or interruption in our ability to meet commercial demand may result in the loss of potential revenues and could adversely affect our ability to gain market acceptance for approved products. In addition, any delay or interruption in the supply of clinical trial supplies could delay the completion of clinical trials, increase the costs associated with maintaining clinical trial programs and, depending upon the period of delay, require us to commence new clinical trials at additional expense or terminate clinical trials completely. Additionally, if supply from one approved manufacturer is interrupted, there could be a significant disruption in commercial supply. Regulatory agencies may also require additional studies if a new manufacturer is relied upon for commercial production. Switching manufacturers may involve substantial costs and is likely to result in a delay in our desired clinical and commercial timelines.
41
We may not be successful in establishing or maintaining development and commercialization collaborations, and any partner may not devote sufficient resources to the development or commercialization of our drug candidates or may otherwise fail in development or commercialization efforts, which could adversely affect our ability to develop certain of our drug candidates and our financial condition and operating results.
Because developing pharmaceutical products, conducting clinical trials, obtaining regulatory approval, establishing manufacturing capabilities and marketing approved products are expensive, we are exploring collaborations with third parties outside of the United States that have more resources and experience. In situations where we enter into a development and commercial collaboration arrangement for a drug candidate, we may also seek to establish additional collaborations for development and commercialization in territories outside of those addressed by the first collaboration arrangement for such drug candidate. There are a limited number of potential partners, and we expect to face competition in seeking appropriate partners. If we are unable to enter into any development and commercial collaborations and/or sales and marketing arrangements on acceptable terms, if at all, we may be unable to successfully develop and seek regulatory approval for our drug candidates and/or effectively market and sell future approved drug products, if any, in all of the territories outside of the United States where it may otherwise be valuable to do so.
Even if we are able to establish collaboration arrangements, any such collaboration may not ultimately be successful, which could have a negative impact on our business, results of operations, financial condition and prospects. If we partner with a third-party for development and commercialization of a drug candidate, we can expect to relinquish some or all of the control over the future success of that drug candidate to the third-party. It is possible that a partner may not devote sufficient resources to the development or commercialization of our drug candidate or may otherwise fail in development or commercialization efforts, in which event the development and commercialization of such drug candidate could be delayed or terminated, and our business could be substantially harmed. In addition, the terms of any collaboration or other arrangement that we establish may not prove to be favorable to us or may not be perceived as favorable, which may negatively impact the trading price of our common shares. In some cases, we may be responsible for continuing development of a product candidate or research program under a collaboration, and the payment we receive from our partner may be insufficient to cover the cost of this development. Moreover, collaborations and sales and marketing arrangements are complex and time consuming to negotiate, document and implement, and they may require substantial resources to maintain.
We are subject to a number of additional risks associated with our dependence on collaborations with third parties, the occurrence of which could cause our collaboration arrangements to fail. Conflicts may arise between us and our partners, such as conflicts concerning the interpretation of clinical data, the achievement of milestones, the interpretation of financial provisions or the ownership of intellectual property developed during the collaboration. If any such conflicts arise, a partner could act in its own self-interest, which may be adverse to our interests. Any such disagreement between us and a partner could result in one or more of the following, each of which could delay or prevent the development or commercialization of our drug candidates and harm our business:
Risks Related to Tax
There is a significant risk that we may be classified as a PFIC for U.S. federal income tax purposes.
Current or potential investors in our common shares who are U.S. Holders (as defined below) should be aware that, based on our most recent financial statements and projections and given uncertainty regarding the composition of our future income and assets, there is a significant risk that we may have been classified as a "passive foreign investment company" or "PFIC" for the 2023 taxable year and may be classified as a PFIC for our current taxable year and possibly subsequent years. Each current or potential investor who is a U.S. Holder should consult his, her or its own tax advisor regarding the U.S. federal, state and local, and non-U.S. tax consequences of the acquisition, ownership, and disposition of our common shares, the U.S. federal tax consequences of the PFIC rules, and the availability of any election that may be available to the holder to mitigate adverse U.S. federal income tax consequences of holding shares of a PFIC.
The rules governing PFICs can have adverse tax effects on U.S. shareholders, which effects may be mitigated by making certain elections for U.S. federal income tax purposes, which elections may or may not be available. If we are a PFIC in any year, a U.S. shareholder in such year will be required to file an annual information return with the IRS on IRS Form 8621 regarding distributions received on their common shares, any gain realized on disposition of such common shares and any other information required by such form. Additionally, if we are classified as a PFIC in any taxable year with respect to which a U.S. shareholder owns common shares, we generally will
42
continue to be treated as a PFIC with respect to such U.S. shareholder in all succeeding taxable years, regardless of whether we continue to meet the tests described above, unless the U.S. shareholder makes a “deemed sale election.”
We may not be able to use our net operating loss carry forwards to offset future taxable income for Canadian or U.S. federal income tax purposes.
At March 31, 2024, Acasti Pharma U.S. had net operating loss carry forwards (“NOLs”) for U.S. federal income tax purposes of approximately $15.4 million, which have no expiry.
Acasti Pharma U.S. underwent an “ownership change” within the meaning of Section 382 of the Code as a result of the merger with Grace Therapeutics, and therefore Acasti Pharma U.S. may become subject to an annual limit on the amount of NOLs that may be used to offset future taxable income of Acasti Pharma U.S. for U.S. federal income tax purposes. Such annual limit is generally equal to the product of (i) the total value of the loss company’s (in this case, Acasti Pharma U.S.) outstanding equity immediately prior to an “ownership change” (subject to certain adjustments); and (ii) the applicable federal long-term tax-exempt interest rate for the month that includes the “ownership change.”
At March 31, 2024, we had NOLs for Canadian federal income tax purposes of approximately $130.1 million, which expire at various dates through 2043. The extent to which we can utilize any or all of our NOLs will depend on many factors, including the jurisdiction applicable to any of our future taxable revenue. In connection with our planned shift of our operations to the United States, we may not be able to justify the allocation of revenue to Canada sufficient to recover the tax benefits arising from NOLs and other tax credits.
Our ability to use NOLs will also depend on the amount of taxable income generated in future periods. The NOLs may expire before we can generate sufficient taxable income to use the NOLs.
The IRS may not agree that we should be treated as a foreign Company for U.S. federal tax purposes.
Although we are incorporated in Quebec, Canada, the IRS may assert that we should be treated as a U.S. Company (and, therefore, a U.S. tax resident) for U.S. federal tax purposes pursuant to Section 7874 of the U.S. Internal Revenue Code of 1986, as amended (the "Code"). For U.S. federal tax purposes, a Company generally is considered a tax resident in the jurisdiction of its organization or incorporation. Because we are an entity incorporated in Canada, we would generally be classified as a foreign Company (and, therefore, not a U.S. tax resident) for U.S. federal tax purposes. Section 7874 of the Code provides an exception under which a foreign Company may, in certain circumstances, be treated as a U.S. Company for U.S. federal tax purposes.
Under Section 7874, if (1) former Grace Therapeutics shareholders owned (within the meaning of Section 7874) 80% or more (by vote or value) of our ordinary shares after the merger by reason of holding Grace Therapeutics common stock (such ownership percentage, the "Section 7874 ownership percentage"), and (2) our "expanded affiliated group" did not have "substantial business activities" in Canada ("the substantial business activities test"), we will be treated as a U.S. Company for U.S. federal tax purposes. If the Section 7874 ownership percentage of the former Grace Therapeutics shareholders after the merger was less than 80% but greater than or equal to 60%, and the substantial business activities test was not met, we and our U.S. affiliates may, in some circumstances, be subject to certain adverse U.S. federal income tax provisions (which, among other things, could limit their ability to utilize certain U.S. tax attributes such as NOLs to offset U.S. taxable income or gain resulting from certain transactions). The application of these rules could result in significant additional U.S. tax liability and limit our ability to restructure or access cash earned by certain of our non-U.S. subsidiaries, in each case, without incurring substantial U.S. tax liabilities.
Based on the terms of the merger, the rules for determining share ownership under Section 7874 and certain factual assumptions, we believe that former Grace Therapeutics shareholders owned (within the meaning of Section 7874) less than 60% (by both vote and value) of our ordinary shares after the merger by reason of holding shares of Grace Therapeutics common stock. Therefore, under current law, we believe that we should not be treated as a U.S. Company for U.S. federal tax purposes and that Section 7874 should otherwise not apply to us or our affiliates as a result of the merger with Grace Therapeutics.
Risks Relating to Our Common Shares
We do not expect to pay any cash dividends for the foreseeable future.
The continued operation and expansion of our business will require substantial funding. Accordingly, we do not anticipate that we will pay any cash dividends on our common shares for the foreseeable future. Any determination to pay dividends in the future will be at the discretion of our board of directors and will depend upon our results of operations, financial condition, contractual restrictions, restrictions imposed by applicable law and other factors our board of directors deems relevant.
The price of our common shares may be volatile.
43
Market prices for securities of pharmaceutical companies can fluctuate significantly. Factors such as the announcement to the public or in various scientific or industry forums of technological innovations; new commercial products; patents or exclusive rights obtained by us or others; disputes or other developments relating to proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our technologies; the commencement, enrollment or announcement of results of clinical trials we conduct, or changes in the development status of our drug candidates; results or delays of pre-clinical and clinical studies by us or others; any delay in our regulatory filings for our drug candidates and any adverse development or perceived adverse development with respect to the applicable regulatory authority’s review of such filings; a change of regulations; additions or departures of key scientific or management personnel; overall performance of the equity markets; general political and economic conditions; publications; failure to meet the estimates and projections of the investment community or that we may otherwise provide to the public; research reports or positive or negative recommendations or withdrawal of research coverage by securities analysts; actual or anticipated variations in quarterly operating results; announcements of significant acquisitions, strategic partnerships, joint ventures or capital commitments by us or our competitors; public concerns over the risks of pharmaceutical products and dietary supplements; unanticipated serious safety concerns related to the use of our drug candidates or drug products; our access to financial resources, future sales of securities by us or our shareholders; and many other factors, many of which are beyond our control, could have considerable effects on the price of our common shares. The price of our common shares has fluctuated significantly in the past and there can be no assurance that the market price of our common shares will not experience significant fluctuations in the future.
In addition, securities of pharmaceutical companies often experience extreme price and volume fluctuations that are unrelated or disproportionate to the operating performance of those companies. Broad market and industry factors may negatively affect the market price of our common shares, regardless of our actual operating performance. In the past, securities class action litigation has often been instituted against pharmaceutical companies following periods of volatility in the market price of their securities. This type of litigation, if instituted against us, could result in substantial costs and a diversion of management’s attention and resources, which would harm our business, operating results or financial condition.
Raising additional capital may cause dilution to our existing shareholders, restrict our operations, or require us to relinquish rights to our technologies or drug candidates.
We will need to raise additional capital in the future in order to fully execute on our business plan. We may seek additional capital through a combination of public and private equity offerings, debt financings, and non-dilutive strategic partnerships and alliances and licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interests of our shareholders will be diluted, and the terms may include liquidation or other preferences that adversely affect the rights of our shareholders. The incurrence of indebtedness by us would result in increased fixed payment obligations and could involve certain restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. If we raise additional funds through strategic partnerships and alliances and licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies or drug candidates, or grant licenses on terms unfavorable to us.
If we fail to meet applicable listing requirements, Nasdaq may delist our common shares from trading, in which case the liquidity and market price of our common shares could decline.
Our common shares are currently listed on the Nasdaq Stock Market LLC (“Nasdaq”), but we cannot assure you that our securities will continue to be listed on Nasdaq in the future. On July 27, 2022, we received written notification from the Nasdaq Listing Qualifications Department for failing to maintain a minimum bid price of $1.00 per common share for the last 30 consecutive business days, as required by Nasdaq Listing Rule 5550(a)(2) - bid price (the “Minimum Bid Price Rule”). The Nasdaq notification had no immediate effect on the listing of our common shares, and we had 180 calendar days, or until January 23, 2023, to regain compliance.
On January 24, 2023, we received notification from Nasdaq that we were eligible for an additional 180 calendar days, or until July 24, 2023, to regain compliance with the Minimum Bid Price Rule. We were granted the second extension because we meet the continued listing requirements for the market value of publicly held shares and all other initial listing standards for Nasdaq, except for the bid price requirement.
On July 24, 2023, we received notification from Nasdaq that we had regained compliance with the minimum bid price requirement set forth in Nasdaq Listing Rule 5550(a)(2) for continued listing on Nasdaq. The notification was sent following the implementation of a 1-for-6 reverse split of our common shares which became effective on July 10, 2023.
If we fail to comply with listing standards and Nasdaq delists our common shares, we and our shareholders could face significant material adverse consequences, including:
44
We may pursue opportunities or transactions that adversely affect our business and financial condition.
Our management, in the ordinary course of our business, regularly explores potential strategic opportunities and transactions. These opportunities and transactions may include strategic joint venture relationships, significant debt or equity investments in us by third parties, the acquisition or disposition of material assets, the licensing, acquisition or disposition of material intellectual property, the development of new drug candidates, the sale of our common shares and other similar opportunities and transactions. The public announcement of any of these or similar strategic opportunities or transactions might have a significant effect on the price of our common shares. Our policy is to not publicly disclose the pursuit of a potential strategic opportunity or transaction unless we are required to do so by applicable law, including applicable securities laws relating to periodic disclosure obligations. There can be no assurance that investors who buy or sell common shares are doing so at a time when we are not pursuing a particular strategic opportunity or transaction that, when announced, would have a significant effect on the price of our common shares.
In addition, any such future corporate development may be accompanied by certain risks, including exposure to unknown liabilities of the strategic opportunities and transactions, higher than anticipated transaction costs and expenses, the difficulty and expense of integrating operations and personnel of any acquired companies, disruption of our ongoing business, diversion of management’s time and attention, and possible dilution to shareholders. We may not be able to successfully overcome these risks and other problems associated with any future acquisitions and this may adversely affect our business and financial condition.
We are a Québec incorporated company, and U.S. investors may be unable to enforce certain judgments against us.
We are a company existing under the Business Corporations Act (Québec), and certain of our assets are located outside the United States. As a result, it may be difficult to effect service within the United States upon us. Execution by U.S. courts of any judgment obtained against us in U.S. courts may be limited to assets located in the United States. It may also be difficult for holders of our securities who reside in the United States to realize in the United States upon judgments of U.S. courts predicated upon civil liability of us under the U.S. federal securities laws. There may be doubt as to the enforceability in Canada against non-U.S. entities, in original actions or in actions for enforcement of judgments of U.S. courts, of liabilities predicated solely upon U.S. federal or state securities laws.
45
Item 1B. Unresolved Staff Comments
Not applicable.
Item 1C. Cybersecurity
We are increasingly dependent on third-party provided software applications and computing infrastructure to conduct key operations. We depend on both our own procured systems, networks, and technology as well as the systems, networks and technology of our contractors, consultants, vendors and other business partners.
Given the importance of cybersecurity to our business, we maintain a robust cybersecurity program as well as cybersecurity policies and processes to support our controls and our preparedness for treatment of identified information security risks. We also undergo annual evaluations of our cybersecurity program, conducted by our cybersecurity external advisory firm.
Our cybersecurity program evaluation identified various risks and issues that we continue to mitigate to further improve our program. This includes:
Process for Assessing, Identifying and Managing Material Risks from Cybersecurity Threats
In the event of a cybersecurity incident, we maintain a Cybersecurity Incident Response Plan. Pursuant to the plan and its escalation protocols, designated personnel are responsible for assessing the severity of an incident and associated threat, containing the threat, remediating the threat, including recovery of data and access to systems, analyzing any reporting obligations associated with the incident, and performing post-incident analysis and program enhancements. We have a relationship with various law firms to assist with advisory on legal aspects of containing incidents and communicating accordingly.
Governance
Management Oversight
The controls and processes employed to assess, identify and manage material risks from cybersecurity threats are implemented and overseen by our information technology (“IT”) contractor. Our IT consultant leverages their over 35 years of experience. Our IT consultant is responsible for the day-to-day management of the cybersecurity program, including the prevention, detection, investigation, response to, and recovery from cybersecurity incidents.
Board Oversight
While our Board of Directors (the “Board”) has overall responsibility for risk oversight, the Audit Committee of the Board (the “Audit Committee”) oversees cybersecurity risk matters. The Audit Committee is responsible for reviewing, monitoring, reporting and, where appropriate, providing recommendations to the Board regarding compliance with our internal policies and its progress in remedying any material deficiencies, including those related to our security policies, including the physical safeguarding of corporate assets and security of our networks and information systems. The Audit Committee receives quarterly updates regarding the cybersecurity program, including top threats and risks, and updates on the cybersecurity roadmap.
Cybersecurity Risks
We maintain a Risk Management Policy that governs the process in which we identify cybersecurity risks, and quantify and evaluate their associated impacts and risk levels. A Cybersecurity Risk Register is also leveraged to track identified cybersecurity risks to date and update treatment of such risks accordingly.
46
For additional information, see “Item 1A—Risk Factors.” In the last two reporting years, we did not experience any material cybersecurity incidents or threats.
Item 2. Properties
Our head office and operations are located at 103 Carnegie Center Suite 300 Princeton, New Jersey, 08540.
Item 3. Legal Proceedings
In the ordinary course of business, we are at times subject to various legal proceedings and disputes. We assess our liabilities and contingencies in connection with outstanding legal proceedings utilizing the latest information available. Where it is probable that we will incur a loss and the amount of the loss can be reasonably estimated, we record a liability in our consolidated financial statements. These legal reserves may be increased or decreased to reflect any relevant developments on a quarterly basis. Where a loss is not probable or the amount of loss is not estimable, we do not accrue legal reserves. While the outcome of legal proceedings is inherently uncertain, based on information currently available and available insurance coverage, our management believes that it has established appropriate legal reserves. Any incremental liabilities arising from pending legal proceedings are not expected to have a material adverse effect on our financial position, results of operations, or cash flows. However, it is possible that the ultimate resolution of these matters, if unfavorable, may be material to our financial position, results of operations, or cash flows. We are not currently a party to any legal proceedings that, in the opinion of management, are likely to have a material adverse effect on our business.
Item 4. Mine Safety Disclosures
Not applicable.
47
PART II
Item 5. Market for Registrant’s Common Equity, Related Shareholder Matters and Issuer Purchases of Equity Securities Market Information
Our common shares are traded on the Nasdaq Capital Market under the symbol “ACST.”
Holders
As of June 21, 2024, there were 95 holders of record of our common shares. The actual number of our shareholders is greater than this number of record holders because most of our shareholders are beneficial owners whose shares are held in street name by brokers and other nominees.
Dividends
We do not anticipate paying any cash dividend on our common shares in the foreseeable future. We presently intend to retain any future earnings to finance the expansion and growth of our business. Any future determination to pay dividends will be at the discretion of our board of directors and will depend on our financial condition, results of operations, capital requirements and other factors the board of directors deems relevant. In addition, the terms of any future debt or credit facility may preclude us from paying dividends.
Taxation
The following is a summary of certain U.S. federal income tax considerations arising from and relating to the acquisition, ownership, and disposition of our common shares to a U.S. Holder (as defined below) as capital assets.
This summary provides only general information and does not purport to be a complete analysis or listing of all potential U.S. federal income tax consequences that may apply to a U.S. Holder as a result of the acquisition, ownership, and disposition of our common shares. In addition, this summary does not take into account the individual facts and circumstances of any particular U.S. Holder that may affect the U.S. federal income tax consequences applicable to that U.S. Holder. Accordingly, this summary is not intended to be, and should not be construed as, legal or U.S. federal income tax advice with respect to any U.S. Holder. Each U.S. Holder should consult its own tax advisor regarding the U.S. federal, state and local, and non-U.S. tax consequences arising from or relating to the acquisition, ownership, and disposition of our common shares.
No legal opinion from U.S. legal counsel or ruling from the IRS, has been requested, or will be obtained, regarding the U.S. federal income tax consequences to U.S. Holders of the acquisition, ownership, and disposition of our common shares. This summary is not binding on the IRS, and the IRS is not precluded from taking a position that is different from, and contrary to, the positions taken in this summary. In addition, because the authorities on which this summary is based are subject to various interpretations, the IRS and the U.S. courts could disagree with one or more of the positions taken in this summary.
Scope of this Disclosure
Authorities
This summary is based on the Code, U.S. Treasury Regulations promulgated thereunder (whether final, temporary or proposed), published IRS rulings, judicial decisions, published administrative positions of the IRS, and the Convention between Canada and the United States of America with Respect to Taxes on Income and on Capital, signed September 26, 1980, as amended (the Canada-U.S. Tax Treaty), in each case, as in effect as of the date of this report. Any of the authorities on which this summary is based could be changed in a material and adverse manner at any time, and any such change could be applied on a retroactive basis. Unless otherwise discussed, this summary does not discuss the potential effects, whether adverse or beneficial, of any proposed legislation.
U.S. Holders
For purposes of this summary, a “U.S. Holder” is a beneficial owner of common shares that, for U.S. federal income tax purposes, is (a) an individual who is a citizen or resident of the United States, (b) a company, or other entity classified as a company for U.S. federal income tax purposes, that is created or organized in or under the laws of the U.S., any state in the United States or the District of Columbia, (c) an estate if the income of such estate is subject to U.S. federal income tax regardless of the source of such income, or (d) a trust if (i) such trust has validly elected to be treated as a U.S. person for U.S. federal income tax purposes or (ii) a U.S. court is able to exercise primary supervision over the administration of such trust and one or more U.S. persons have the authority to control all substantial decisions of such trust.
U.S. Holders Subject to Special U.S. Federal Income Tax Rules Not Addressed
This summary does not address the U.S. federal income tax consequences applicable to U.S. Holders that are subject to special provisions under the Code, including, but not limited to, the following U.S. Holders: (a) U.S. Holders that are tax-exempt organizations, qualified retirement plans, individual retirement accounts, or other tax deferred accounts; (b) U.S. Holders that are financial institutions, insurance
48
companies, real estate investment trusts, or regulated investment companies; (c) U.S. Holders that are dealers in securities or currencies or U.S. Holders that are traders in securities that elect to apply a mark-to-market accounting method; (d) U.S. Holders that have a “functional currency” other than the U.S. dollar; (e) U.S. Holders subject to the alternative minimum tax provisions of the Code; (f) U.S. Holders that own common shares as part of a straddle, hedging transaction, conversion transaction, integrated transaction, constructive sale, or other arrangement involving more than one position; (g) U.S. Holders that acquired common shares through the exercise of employee stock options or otherwise as compensation for services; (h) U.S. Holders that hold common shares other than as a capital asset within the meaning of Section 1221 of the Code; (i) U.S. Holders that beneficially own (directly, indirectly or by attribution) 10% or more of our equity securities (by vote or value); and (j) U.S. expatriates. U.S. Holders that are subject to special provisions under the Code, including U.S. Holders described above, should consult their own tax advisor regarding the U.S. federal, U.S. federal alternative minimum, U.S. federal estate and gift, U.S. state and local, and non-U.S. tax consequences arising from and relating to the acquisition, ownership, and disposition of the common shares.
If an entity or arrangement that is classified as a partnership for U.S. federal income tax purposes holds common shares, the U.S. federal income tax consequences to that partnership and the partners of that partnership generally will depend on the activities of the partnership and the status of the partners. Partners of entities that are classified as partnerships for U.S. federal income tax purposes should consult their own tax advisors regarding the U.S. federal income tax consequences arising from and relating to the acquisition, ownership and disposition of the common shares.
Tax Consequences Other than U.S. Federal Income Tax Consequences Not Addressed
This summary does not address the U.S. estate and gift, alternative minimum, state, local or non-U.S. tax consequences to U.S. Holders of the acquisition, ownership, and disposition of our common shares. Each U.S. Holder should consult its own tax advisor regarding the U.S. estate and gift, alternative minimum, state, local and non-U.S. tax consequences arising from and relating to the acquisition, ownership, and disposition of our common shares.
Other Assumptions
Notwithstanding that Acasti is organized under Québec law, if (1) former Grace Therapeutics shareholders owned (within the meaning of Section 7874 of the Code) 80% or more (by vote or value) of our ordinary shares after the merger by reason of holding Grace Therapeutics common stock (such ownership percentage, the “Section 7874 ownership percentage”), and (2) our “expanded affiliated group” did not have “substantial business activities" in Canada (“the substantial business activities test”), we will be treated as a U.S. company for U.S. federal tax purposes. If the Section 7874 ownership percentage of the former Grace Therapeutics shareholders after the merger was less than 80% but greater than or equal to 60%, and the substantial business activities test was not met, we and our U.S. affiliates may, in some circumstances, be subject to certain adverse U.S. federal income tax provisions (which, among other things, could limit their ability to utilize certain U.S. tax attributes such as NOLs to offset U.S. taxable income or gain resulting from certain transactions).
Based on the terms of the merger, the rules for determining share ownership under Section 7874 of the Code and certain factual assumptions, we believe that former Grace Therapeutics shareholders owned (within the meaning of Section 7874 of the Code) less than 60% (by both vote and value) of our ordinary shares after the merger by reason of holding shares of Grace Therapeutics common stock. Therefore, under current law, we believe that we should not be treated as a U.S. company for U.S. federal tax purposes and that Section 7874 of the Code should otherwise not apply to us or our affiliates as a result of the merger with Grace Therapeutics. The remainder of the discussion in this section, “Taxation”, assumes that Acasti is and at all times has been properly classified as a non-U.S. company for U.S. federal income tax purposes.
U.S. Federal Income Tax Considerations of the Acquisition, Ownership, and Disposition of Common Shares
Distributions on Common Shares
Subject to the discussion under “—Passive Foreign Investment Company Rules” below, a U.S. Holder that receives a distribution, including a constructive distribution or a taxable stock distribution, with respect to the common shares generally will be required to include the amount of that distribution in gross income as a dividend (without reduction for any Canadian income tax withheld from such distribution) to the extent of our current or accumulated “earnings and profits,” (as computed for U.S. federal income tax purposes). To the extent that a distribution exceeds our current and accumulated “earnings and profits”, the excess amount will be treated (a) first, as a tax-free return of capital to the extent of a U.S. Holder’s adjusted tax basis in the common shares with respect to which the distribution is made (resulting in a corresponding reduction in the tax basis of those common shares) and, (b) thereafter, as gain from the sale or exchange of those common shares (see the more detailed discussion at “—Disposition of Common Shares” below). We do not intend to calculate our current or accumulated earnings and profits for U.S. federal income tax purposes and, therefore, will not be able to provide U.S. Holders with that information. U.S. Holders should therefore assume that any distribution by us with respect to our common shares will constitute a dividend. However, U.S. Holders should consult their own tax advisors regarding whether distributions from us should be treated as dividends for U.S. federal income tax purposes. Dividends paid on our common shares generally will not be eligible for the “dividends received deduction” allowed to companies under the Code with respect to dividends received from U.S. companies.
49
A dividend paid by us generally will be taxed at the preferential tax rates applicable to long-term capital gains if, among other requirements, (a) we are a “qualified foreign company” (as defined below), (b) the U.S. Holder receiving the dividend is an individual, estate, or trust, and (c) the dividend is paid on common shares that have been held by the U.S. Holder for at least 61 days during the 121-day period beginning 60 days before the “ex-dividend date” (i.e., the first date that a purchaser of the common shares will not be entitled to receive the dividend).
For purposes of the rules described in the preceding paragraph, we generally would be a “qualified foreign Company”(“QFC”), if (a) we are eligible for the benefits of the Canada-U.S. Tax Treaty, or (b) our common shares are readily tradable on an established securities market in the United States, within the meaning provided in the Code. However, even if we satisfy one or more of the requirements, we will not be treated as a QFC if we are classified as a PFIC (as discussed below) for the taxable year during which we pay the applicable dividend or for the preceding taxable year. The dividend rules are complex, and each U.S. Holder should consult its own tax advisor regarding the application of those rules to them in their particular circumstances. Even if we satisfy one or more of the requirements, as noted below. Thus, there can be no assurance that we will qualify as a QFC.
Disposition of Common Shares
Subject to the discussion under “—Passive Foreign Investment Company Rules” below, a U.S. Holder will recognize gain or loss on the sale or other taxable disposition of common shares (that is treated as a sale or exchange for U.S. federal income tax purposes) equal to the difference, if any, between (a) the U.S. dollar value of the amount realized on the date of the sale or disposition and (b) the U.S. Holder’s adjusted tax basis (determined in U.S. dollars) in the common shares sold or otherwise disposed of. Any such gain or loss generally will be capital gain or loss, which will be long-term capital gain or loss if the common shares are held for more than one year. A U.S. Holder's initial tax basis in the common shares generally will equal the U.S. dollar cost of such common shares. Each U.S. Holder should consult its own tax advisor as to the tax treatment of dispositions of common shares in exchange for Canadian dollars.
Subject to the discussion under “—Passive Foreign Investment Company Rules” below, preferential tax rates apply to long-term capital gains of a U.S. Holder that is an individual, estate, or trust. There are currently no preferential tax rates for long-term capital gains of a U.S. Holder that is a company. Deductions for capital losses are subject to complex limitations.
Passive Foreign Investment Company Rules
If we are or become a PFIC, the preceding sections of this summary may not describe the U.S. federal income tax consequences to U.S. Holders of the acquisition, ownership, and disposition of our common shares.
Passive Foreign Investment Company Status.
Special, generally unfavorable, rules apply to the ownership and disposition of the stock of a PFIC. For U.S. federal income tax purposes, a non-U.S. company is classified as a PFIC if:
Passive income generally includes the following types of income:
In determining whether we are a PFIC, we will be required to take into account a pro rata portion of the income and assets of each company in which we own, directly or indirectly, at least 25% by value.
As described above, PFIC status of a non-U.S. company depends on the relative values of certain categories of assets and the relative amount of certain kinds of income for a taxable year. Therefore, our status as a PFIC for any given taxable year depends upon the financial results for such year and upon relative valuations, which are subject to change and beyond our ability to predict or control. Based on our most recent financial statements and projections and given uncertainty regarding the composition of our future income and assets, there is a significant risk that we may have been classified as a PFIC for the taxable year that ended on March 31, 2024 and may be classified as a PFIC for our current taxable year and possibly subsequent years. However, PFIC status is fundamentally factual in nature, depends on the application of complex U.S. federal income tax rules (which are subject to differing interpretations), generally cannot be determined until the close of the taxable year in question and is determined annually. Accordingly, there can be no assurance that we will not be a PFIC in our current taxable year or subsequent years. The PFIC rules are complex, and each U.S. Holder should consult its tax advisor regarding the application of the PFIC rules to us.
50
Default PFIC Rules Under Section 1291 of the Code.
Generally, if we are or have been treated as a PFIC for any taxable year during a U.S. Holder’s holding period of common shares, subject to the special rules described below applicable to a U.S. Holder who makes a Mark-to-Market Election or a QEF Election (each as defined below), any “excess distribution” with respect to the common shares would be allocated ratably over the U.S. Holder’s holding period. The amounts allocated to the taxable year of the excess distribution and to any year before we became a PFIC would be taxed as ordinary income. The amount allocated to each other taxable year would be subject to tax at the highest rate in effect for individuals or companies in that taxable year, as appropriate, and an interest charge would be imposed on the amount allocated to that taxable year. Distributions made in respect of common shares during a taxable year will be excess distributions to the extent they exceed 125% of the average of the annual distributions on common shares received by the U.S. Holder during the preceding three taxable years or the U.S. Holder’s holding period, whichever is shorter. In addition, dividends generally will not be qualified dividend income if we are a PFIC in the taxable year of payment or the preceding year.
Generally, if we are treated as a PFIC for any taxable year during which a U.S. Holder owns common shares, any gain on the disposition of the common shares would be treated as an excess distribution and would be allocated ratably over the U.S. Holder’s holding period and subject to taxation in the same manner as described in the preceding paragraph and would not be eligible for the preferential long-term capital gains rate.
Certain elections (including the Mark-to-Market Election and the QEF Election, as defined and discussed below) may sometimes be used to mitigate the adverse impact of the PFIC rules on U.S. Holders, but these elections may accelerate the recognition of taxable income and have other adverse consequences.
Each current or prospective U.S. Holder should consult its own tax advisor regarding potential status of us as a PFIC, the possible effect of the PFIC rules to such holder in his, her or its particular circumstances, information reporting required if we were treated as a PFIC and the availability of any election that may be available to the U.S. holder to mitigate adverse U.S. federal income tax consequences of holding shares in a PFIC.
QEF Election.
A U.S. Holder of common shares in a PFIC generally would not be subject to the PFIC rules discussed above if the U.S. Holder had made a timely and effective election (a “QEF Election”) to treat us as a “qualified electing fund” (a “QEF”). Instead, such U.S. Holder would be subject to U.S. federal income tax on its pro rata share of our (i) net capital gain, which would be taxed as long-term capital gain to such U.S. Holder, and (ii) ordinary earnings, which would be taxed as ordinary income to such U.S. Holder, in each case regardless of whether such amounts are actually distributed to such U.S. Holder. However, a U.S. Holder that makes a QEF Election may, subject to certain limitations, elect to defer payment of current U.S. federal income tax on such amounts, subject to an interest charge. If such U.S. Holder is not a company, any such interest paid will be treated as “personal interest,” which is not deductible.
A U.S. Holder that makes a timely and effective QEF Election generally (a) may receive a tax-free distribution from us to the extent that such distribution represents our “earnings and profits” that were previously included in income by such U.S. Holder because of such QEF Election and (b) will adjust such U.S. Holder’s tax basis in the common shares to reflect the amount included in income or allowed as a tax-free distribution because of such QEF Election. In addition, for U.S. federal income tax purposes, a U.S. Holder that makes a timely QEF Election generally will recognize capital gain or loss on the sale or other taxable disposition of the common shares.
A QEF Election will be treated as “timely” if such QEF Election is made for the first taxable year in the U.S. Holder’s holding period for the common shares in which we are a PFIC. A U.S. Holder may make a timely QEF Election by filing the appropriate QEF Election documents at the time such U.S. Holder files a U.S. federal income tax return for such first year. If a U.S. Holder makes a QEF Election after the first taxable year in the U.S. Holder’s holding period for the common shares in which we are a PFIC, then, in addition to filing the QEF Election documents, a U.S. Holder may elect to recognize gain (which will be taxed under the rules discussed under “—Default PFIC Rules Under Section 1291 of the Code”) as if the common shares were sold on the qualification date. The “qualification date” is the first day of the first taxable year in which we are a QEF with respect to such U.S. Holder. The election to recognize such gain can only be made if such U.S. Holder’s holding period for the common shares includes the qualification date. By electing to recognize such gain, such U.S. Holder will be deemed to have made a timely QEF Election. In addition, under very limited circumstances, it is possible that a U.S. Holder might make a retroactive QEF Election if such U.S. Holder failed to file the QEF Election documents in a timely manner. If a U.S. Holder fails to make a QEF Election for the first taxable year in the U.S. Holder’s holding period for the common shares in which we are a PFIC and does not elect to recognize gain as if the common shares were sold on the qualification date, such holder will not be treated as having made a “timely” QEF Election and will continue to be subject to the special adverse taxation rules discussed above under “—Default PFIC Rules Under Section 1291 of the Code.”
A QEF Election will apply to the taxable year for which such QEF Election is made and to all subsequent taxable years, unless such QEF Election is invalidated or terminated or the IRS consents to revocation of such QEF Election. If a U.S. Holder makes a QEF Election and, in a subsequent taxable year, we cease to be a PFIC, the QEF Election will remain in effect (although it will not be applicable) during those taxable years in which we are not a PFIC. Accordingly, if we become a PFIC in another subsequent taxable
51
year, the QEF Election will be effective, and the U.S. Holder will be subject to the rules described above during any such subsequent taxable year in which we qualify as a PFIC.
A U.S. Holder cannot make and maintain a valid QEF Election unless we provide certain U.S. tax information necessary to make such an election. On an annual basis, we intend to use commercially reasonable efforts to make available to U.S. Holders, upon their written request (a) timely information as to our status as a PFIC, and (b) for each year in which we are a PFIC, information and documentation that a U.S. Holder making a QEF Election with respect to us is required to obtain for U.S. federal income tax purposes. Each U.S. Holder should consult its own tax advisor regarding the availability of, and procedure for making, a QEF Election with respect to us.
Mark-to-Market Election.
A U.S. Holder of common shares in a PFIC would not be subject to the PFIC rules discussed above under “—Default PFIC Rules Under Section 1291 of the Code” if the U.S. Holder had made a timely and effective election to mark the PFIC common shares to market (a “Mark-to-Market Election”).
A U.S. Holder may make a Mark-to-Market Election with respect to the common shares only if such shares are marketable stock. Such shares generally will be “marketable stock” if they are regularly traded on a “qualified exchange,” which is defined as (a) a national securities exchange that is registered with the SEC, (b) the national market system established pursuant to section 11A of the Exchange Act, or (c) a non-U.S. securities exchange that is regulated or supervised by a governmental authority of the country in which the market is located, provided that (i) such non-U.S. exchange has trading volume, listing, financial disclosure, surveillance, and other requirements, and the laws of the country in which such non-U.S. exchange is located, together with the rules of such non-U.S. exchange, ensure that such requirements are actually enforced and (ii) the rules of such non-U.S. exchange ensure active trading of listed stocks. Our common shares will generally be treated as “regularly traded” in any calendar year in which more than a de minimis quantity of common shares is traded on a qualified exchange for at least 15 days during each calendar quarter. Each U.S. Holder should consult its own tax advisor with respect to the availability of a Mark-to-Market Election with respect to the common shares.
In general, a U.S. Holder that makes a timely Mark-to-Market Election with respect to the common shares will include in ordinary income, for each taxable year in which we are a PFIC, an amount equal to the excess, if any, of (a) the fair market value of the common shares as of the close of such taxable year over (b) such U.S. Holder’s tax basis in such shares. A U.S. Holder that makes a Mark-to-Market Election will be allowed a deduction in an amount equal to the lesser of (a) the excess, if any, of (i) such U.S. Holder’s adjusted tax basis in the common shares over (ii) the fair market value of such shares as of the close of such taxable year or (b) the excess, if any, of (i) the amount included in ordinary income because of such Mark-to-Market Election for prior taxable years over (ii) the amount allowed as a deduction because of such Mark-to-Market Election for prior taxable years. If a U.S. Holder makes a Mark-to-Market Election after the first taxable year in which we are a PFIC and such U.S. Holder has not made a timely QEF Election with respect to us, the PFIC rules described above under “—Default PFIC Rules Under Section 1291 of the Code” will apply to certain dispositions of, and distributions on, the common shares, and the U.S. Holder’s mark-to-market income for the year of the election. If we were to cease being a PFIC, a U.S. Holder that marked its common shares to market would not include mark-to-market gain or loss with respect to its common shares for any taxable year that we were not a PFIC.
A U.S. Holder that makes a Mark-to-Market Election generally will also adjust such U.S. Holder’s tax basis in his common shares to reflect the amount included in gross income or allowed as a deduction because of such Mark-to-Market Election. In addition, upon a sale or other taxable disposition of the common shares subject to a Mark-to-Market Election, any gain or loss on such disposition will be ordinary income or loss (to the extent that such loss does not to exceed the excess, if any, of (a) the amount included in ordinary income because of such Mark-to-Market Election for prior taxable years over (b) the amount allowed as a deduction because of such Mark-to-Market Election for prior taxable years). A Mark-to-Market Election applies to the taxable year in which such Mark-to-Market Election is made and to each subsequent taxable year unless the common shares cease to be “marketable stock” or the IRS consents to revocation of such election. Each U.S. Holder should consult its own tax advisor regarding the availability of, and procedure for making, a Mark-to-Market Election with respect to the common shares.
Reporting.
If we were to be treated as a PFIC in any taxable year, a U.S. Holder will generally be required to file an annual report with the IRS containing such information as the U.S. Treasury Department may require.
Each U.S. Holder should consult its own tax advisor regarding our potential status as a PFIC, the possible effect of the PFIC rules to such holder and information reporting required if we were a PFIC, as well as the availability of any election that may be available to the holder to mitigate adverse U.S. federal income tax consequences of holding shares in a PFIC.
Receipt of Foreign Currency
The amount of a distribution paid in Canadian dollars or Canadian dollar proceeds received on the sale or other taxable disposition of common shares will generally be equal to the U.S. dollar value of the currency on the date of receipt. If any Canadian dollars received with respect to the common shares are later converted into U.S. dollars, U.S. Holders may realize foreign currency gain or loss on the
52
conversion. Any gain or loss generally will be treated as ordinary income or loss and generally will be from sources within the United States for U.S. foreign tax credit purposes. Each U.S. Holder should consult its own tax advisor concerning the possibility of foreign currency gain or loss if any such currency is not converted into U.S. dollars on the date of receipt.
Foreign Tax Credit
Subject to certain limitations, a U.S. Holder who pays (whether directly or through withholding) Canadian or other non-U.S. income tax with respect to the common shares may be entitled, at the election of the U.S. Holder, to receive either a deduction or a credit for Canadian or other non-U.S. income tax paid. Dividends paid on common shares generally will constitute income from sources outside the United States. Any gain from the sale or other taxable disposition of the common shares by a U.S. Holder generally will constitute U.S. source income. The foreign tax credit rules (including the limitations with respect thereto) are complex, and each U.S. Holder should consult its own tax advisor regarding the foreign tax credit rules, having regard to such holder’s particular circumstances.
Information Reporting; Backup Withholding
Generally, information reporting and backup withholding will apply to distributions on, and the payment of proceeds from the sale or other taxable disposition of, the common shares unless (i) the U.S. Holder is a company or other exempt entity, or (ii) in the case of backup withholding, the U.S. Holder provides a correct taxpayer identification number, certifies that the U.S. Holder is not subject to backup withholding and otherwise complies with the applicable requirements of the backup withholding rules.
Backup withholding is not an additional tax. Any amount withheld generally will be creditable against a U.S. Holder’s U.S. federal income tax liability or refundable to the extent that it exceeds such liability provided the required information is provided to the IRS in a timely manner.
In addition, certain categories of U.S. Holders must file information returns with respect to their investment in a non-U.S. company. For example, certain U.S. Holders must file IRS Form 8938 with respect to certain “specified foreign financial assets” (such as the common shares) with an aggregate value in excess of US$50,000 (and, in some circumstances, a higher threshold). Failure to do so could result in substantial penalties and in the extension of the statute of limitations with respect to such holder’s U.S. federal income tax returns. Each U.S. Holder should consult its own tax advisor regarding application of the information reporting and backup withholding rules to it in connection with an investment in our common shares.
Medicare Contribution Tax
U.S. Holders that are individuals, estates or certain trusts generally will be subject to a 3.8% Medicare contribution tax on, among other things, dividends on, and capital gains from the sale or other taxable disposition of, common shares, subject to certain limitations and exceptions. Each U.S. Holder should consult its own tax advisor regarding possible application of this additional tax to income earned in connection with an investment in our common shares.
Recent Sales of Unregistered Securities
None.
Issuer Repurchases of Equity Securities
None.
Item 6. Reserved
53
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operation
The following discussion should be read in conjunction with our consolidated financial statements and notes thereto found elsewhere in this Annual Report on Form 10-K. This Annual Report on Form 10-K contains forward-looking statements within the meaning of the U.S. Private Securities Litigation Reform Act of 1995. These statements are subject to risks and uncertainties that could cause actual results and events to differ materially from those expressed or implied by such forward-looking statements. For a detailed discussion of these risks and uncertainties, see Item 1A, “Risk Factors” of this Annual Report on Form 10-K. We caution readers not to place undue reliance on these forward-looking statements, which reflect management’s analysis only as of the date of this Annual Report on Form 10-K. We undertake no obligation to update forward-looking statements which reflect events or circumstances occurring after the date of this Annual Report on Form 10-K, unless required by applicable securities laws.
Overview
This management’s discussion and analysis (“MD&A”) is presented in order to provide the reader with an overview of the financial results and changes to our financial position as at March 31, 2024 and for the year then ended. This MD&A explains the material variations in our operations, financial position and cash flows for the years ended March 31, 2024 and 2023.
Market data, and certain industry data and forecasts included in this MD&A were obtained from internal surveys and market research conducted by third parties hired by us, publicly available information, reports of governmental agencies and industry publications, and independent third-party surveys. We have relied upon industry publications as our primary sources for third-party industry data and forecasts. Industry surveys, publications and forecasts generally state that the information they contain has been obtained from sources believed to be reliable, but that the accuracy and completeness of that information are not guaranteed. We have not independently verified any of the data from third-party sources or the underlying economic assumptions they have made. Similarly, internal surveys, industry forecasts and market research, which we believe to be reliable based upon management or contracted third parties’ knowledge of our industry, have not been independently verified. Our estimates involve risks and uncertainties, including assumptions that may prove not to be accurate, and these estimates and certain industry data are subject to change based on various factors, including those discussed in this Annual Report on Form 10-K.
This MD&A should be read in conjunction with our consolidated financial statements for the years ended March 31, 2024 and 2023 included elsewhere in this Annual Report on Form 10-K. Our annual financial statements were prepared in accordance with U.S. GAAP.
Our assets as at March 31, 2024, include cash and cash equivalents of $23.0 million and intangible assets and goodwill of $49.3 million. Our current liabilities were $1.7 million as of March 31, 2024 and are comprised primarily of amounts due to or accrued for creditors.
Reverse stock split
On June 29, 2023, our Board of Directors approved an amendment to our Articles of Incorporation to implement a reverse stock split of our common shares, at a ratio of 1-for-6 (the “Reverse Stock Split”). On July 4, 2023, we filed Articles of Amendment to our Articles of Incorporation with the Registraire des entreprises du Québec, to implement the Reverse Stock Split. All references in this MD&A to number of common shares, warrants and options, and number of shares outstanding have been adjusted to reflect the Reverse Stock Split, which became effective on July 10, 2023.
54
Results of Operations
Comparison of the year ended March 31, 2024, and 2023
The following table summarizes our results of operations for the years ended March 31, 2024 and 2023:
|
|
Year ended |
|
|
|
|
||||||
|
|
March 31, |
|
|
March 31, |
|
|
Increase (Decrease) |
|
|||
|
|
$ |
|
|
$ |
|
|
$ |
|
|||
|
(in thousands) |
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|||
Operating expenses |
|
|
|
|
|
|
|
|
|
|||
Research and development expenses, net of government assistance |
|
|
4,683 |
|
|
|
9,972 |
|
|
|
(5,289 |
) |
General and administrative expenses |
|
|
6,432 |
|
|
|
7,614 |
|
|
|
(1,182 |
) |
Sales and marketing expenses |
|
|
252 |
|
|
|
661 |
|
|
|
(409 |
) |
Restructuring cost |
|
|
1,485 |
|
|
|
— |
|
|
|
1,485 |
|
Impairment of intangible assets |
|
|
— |
|
|
|
28,682 |
|
|
|
(28,682 |
) |
Impairment of goodwill |
|
|
— |
|
|
|
4,826 |
|
|
|
(4,826 |
) |
Impairment of assets held for sale |
|
|
— |
|
|
|
400 |
|
|
|
(400 |
) |
Loss from operating activities |
|
|
(12,852 |
) |
|
|
(52,155 |
) |
|
|
(39,303 |
) |
|
|
|
|
|
|
|
|
|
|
|||
Foreign exchange loss |
|
|
(16 |
) |
|
|
(72 |
) |
|
|
56 |
|
Change in fair value of derivative warrant liabilities |
|
|
(2,728 |
) |
|
|
10 |
|
|
|
(2,738 |
) |
Interest income and other expense, net |
|
|
911 |
|
|
|
246 |
|
|
|
665 |
|
Income tax benefit |
|
|
1,832 |
|
|
|
9,542 |
|
|
|
(7,710 |
) |
|
|
|
|
|
|
|
|
|
|
|||
Net loss |
|
|
(12,853 |
) |
|
|
(42,429 |
) |
|
|
(29,576 |
) |
Net Loss
The net loss of $12.9 million or $1.35 loss per share for the year ended March 31, 2024, decreased by $29.6 million from the net loss of $42.4 million or $5.71 loss per share for the year ended March 31, 2023. The decrease in net loss was primarily due to asset impairments, net of income tax benefit, totaling $25.3 million during the year ended March 31, 2023.
Research and development expenses
Research and development expenses consist primarily of:
We record research and development expenses as incurred.
Our research and development during the year ended March 31, 2024 was focused primarily on our clinical development programs for our GTX-104 drug candidate. Research and development expenses during the year ended March 31, 2023, were focused primarily on our clinical development programs GTX-104, GTX-102, and GTX-101 drug candidates.
The following table summarizes our research and development expenses:
55
Research and development expenses |
|
|
|
|
|
|
|
|
|
|||
|
|
Year ended |
|
|
|
|
||||||
|
|
March 31, 2024 |
|
|
March 31, 2023 |
|
|
Increase (Decrease) |
|
|||
|
|
$ |
|
|
$ |
|
|
$ |
|
|||
|
(in thousands) |
|
||||||||||
Total third-party research and development expenses1 |
|
|
3,576 |
|
|
|
7,704 |
|
|
|
(4,128 |
) |
Government grants & tax credits |
|
|
55 |
|
|
|
(165 |
) |
|
|
220 |
|
Salaries and benefits |
|
|
844 |
|
|
|
1,742 |
|
|
|
(898 |
) |
Research and development expense before stock-based compensation and depreciation |
|
|
4,475 |
|
|
|
9,281 |
|
|
|
(4,806 |
) |
Stock-based compensation |
|
|
198 |
|
|
|
591 |
|
|
|
(393 |
) |
Depreciation and loss on disposal |
|
|
10 |
|
|
|
100 |
|
|
|
(90 |
) |
Total |
|
|
4,683 |
|
|
|
9,972 |
|
|
|
(5,289 |
) |
1Total third-party research and development expenses are calculated before salaries and benefits, depreciation, write-off of equipment and stock-based compensation. Because there is no standard method endorsed by U.S. GAAP, the results may not be comparable to similar measurements presented by other public companies.
Total third-party research and development expenses for the year ended March 31, 2024, were $3.6 million, compared to $7.7 million for the year ended March 31, 2023. This decrease of $4.1 million was primarily due to the restructuring to align our organizational and management cost structure to prioritize resources to GTX-104, thereby reducing losses to improve cash flow and extend available cash resources. Our clinical development programs for GTX-102, and GTX-101 were de-prioritized in the current year compared to the prior year.
Government grants and tax credits of $55 thousand for the year ended March 31, 2024, decreased by $220 thousand compared to $(165) thousand for the year ended March 31, 2023. The changes within government grants and tax credits were due to adjustments of provisions regarding the estimated realizability of credits receivable after assessments and correspondence from tax authorities.
Salaries and benefits of $844 thousand for the year ended March 31, 2024, decreased by $898 thousand compared to $1.7 million for the year ended March 31, 2023. The decrease was primarily due to a reduction in research and development headcount due to the restructuring.
Stock-based compensation of $198 thousand for the year ended March 31, 2024, decreased by $393 thousand compared to $591 thousand for the year ended March 31, 2023. The decrease was primarily due to a reduction in research and development headcount due to the restructuring.
General and administrative expenses
General and administrative expenses consist primarily of salaries and related benefits, including stock-based compensation, related to our executive, finance, legal, and support functions, including professional fees for auditing, tax, consulting, rent and utilities and insurance.
General and administrative expenses |
|
|
|
|
|
|
|
|
|
|||
|
|
Year ended |
|
|
|
|
||||||
|
|
March 31, 2024 |
|
|
March 31, 2023 |
|
|
Increase (Decrease) |
|
|||
|
|
$ |
|
|
$ |
|
|
$ |
|
|||
|
(in thousands) |
|
||||||||||
Salaries and benefits |
|
|
1,164 |
|
|
|
2,362 |
|
|
|
(1,198 |
) |
Professional fees |
|
|
3,188 |
|
|
|
2,013 |
|
|
|
1,175 |
|
Other |
|
|
1,343 |
|
|
|
2,053 |
|
|
|
(710 |
) |
General and administrative expense before stock-based compensation and depreciation 1 |
|
|
5,695 |
|
|
|
6,428 |
|
|
|
(733 |
) |
Stock-based compensation |
|
|
697 |
|
|
|
1,123 |
|
|
|
(426 |
) |
Depreciation and loss on disposal |
|
|
40 |
|
|
|
63 |
|
|
|
(23 |
) |
Total |
|
|
6,432 |
|
|
|
7,614 |
|
|
|
(1,182 |
) |
1 General and administrative sub-total expenses is calculated before stock-based compensation and depreciation. Because there is no
56
standard method endorsed by U.S. GAAP, the results may not be comparable to similar measurements presented by other public companies.
General and administrative expenses were $5.7 million before stock-based compensation and depreciation expense for the year ended March 31, 2024, a decrease of $733 thousand from $6.4 million for the year ended March 31, 2023. The decrease was primarily a result of decreased salaries and benefits due to a reduction in general and administrative headcount due to our restructuring and reorganization of our management structure offset by increased legal, tax, accounting and other professional fees related to the restructuring. Stock-based compensation of $697 thousand for the year ended March 31, 2024, decreased by $426 thousand compared to $1.1 million for the year ended March 31, 2023. The decrease was primarily due to a reduction in general and administrative headcount as a result of our restructuring.
Sales and marketing
Sales and marketing expenses consist primarily of salaries and benefits, including share-based compensation, related to our commercial functions.
Sales and marketing expenses |
|
|
|
|
|
|
|
|
|
|||
|
|
Year ended |
|
|
|
|
||||||
|
|
March 31, 2024 |
|
|
March 31, 2023 |
|
|
Increase (Decrease) |
|
|||
|
|
$ |
|
|
$ |
|
|
$ |
|
|||
|
(in thousands) |
|
||||||||||
Salaries and benefits |
|
|
20 |
|
|
|
428 |
|
|
|
(408 |
) |
Professional fees |
|
|
20 |
|
|
|
— |
|
|
|
20 |
|
Other |
|
|
194 |
|
|
|
136 |
|
|
|
58 |
|
Sales and Marketing expenses before stock-based compensation1 |
|
|
234 |
|
|
|
564 |
|
|
|
(330 |
) |
Stock-based compensation |
|
|
18 |
|
|
|
97 |
|
|
|
(79 |
) |
Total |
|
|
252 |
|
|
|
661 |
|
|
|
(409 |
) |
1 Sales and marketing sub-total expenses is calculated before stock-based compensation. Because there is no standard method endorsed by U.S. GAAP, the results may not be comparable to similar measurements presented by other public companies.
Sales and marketing expenses before stock-based compensation expense were $234 thousand for the year ended March 31, 2024, compared to $564 thousand for the year ended March 31, 2023. The decrease of $330 thousand was primarily due to the reduction of headcount due to our restructuring and reorganization of our management structure.
Stock-based compensation of $18 thousand for the year ended March 31, 2024, decreased by $79 thousand, compared to $97 thousand for the year ended March 31, 2023. The decrease is primarily due to a reduction in sales and marketing headcount due to our restructuring.
Restructuring Costs
On May 8, 2023, we announced our decision to terminate a substantial amount of our workforce as part of a plan intended to align our organizational and management cost structure to prioritize resources to GTX-104, thereby reducing losses to improve cash flow and extend available cash resources. We incurred $1.5 million of related costs primarily consisting of employee severance costs.
Impairment
In April 2023, we announced the strategic decision to prioritize development of GTX-104 with a goal to advance to commercialization, while conserving resources as much as possible to complete development efficiently. We estimated that the deferral could be 3 years from April 2023, given the timeline to complete the development and commercial launch of GTX-104. Further development of GTX-102 and GTX-101 will occur at such time as we obtain additional funding or enter into strategic partnerships.
The decision to defer further development triggered a comprehensive impairment review of our intangible assets in March 2023. Given the extended timeline, we increased the discount rates used to value the assets in order to recognize additional risks related to prioritizing one asset over the others, financing the projects given limited available resources and the need to preserve cash to advance GTX-104 as far as possible, potential competitor advances that could arise over three years, and the general market depression affecting small cap development companies like us and the prohibitively high dilution and expense of available funding in the capital markets. Increasing the discount rates significantly reduced the discounted cash flow values for each of the programs deferred.
Accordingly, an impairment of intangible assets of $28.7 million resulted in the year ended March 31, 2023, compared to nil for the year ended March 31, 2024. In addition, an impairment of $4.8 million of goodwill resulted in the year ended March 31, 2023, compared
57
to nil for the year ended March 31, 2024.
Change in fair value of derivative warrant liabilities
The increase in fair value of derivative warrant liabilities for the year ended March 31, 2024 of $2.7 million was mainly attributable to an increase in our stock price.
Interest income
Interest income was $911 thousand for the year ended March 31, 2024, compared to $246 thousand for the year ended March 31, 2023. The $665 thousand increase in our interest income was due to higher interest rates earned on average balances of cash and cash equivalents.
Income tax benefit
Income tax benefit was $1.8 million for the year ended March 31, 2024, compared to $9.5 million for the year ended March 31, 2023. In 2023, the impairment of $28.7 million of the intangible assets resulted in an income tax recovery of $8.6 million of the related deferred tax liability. In 2024, there was no such impairment.
Liquidity and Capital Resources
Cash Flows and Financial Condition between the years ended March 31, 2024 and March 31, 2023
Summary
As of March 31, 2024, cash and cash equivalents were $23.0 million, a net decrease of $4.9 million compared to cash and cash equivalents of $27.9 million at March 31, 2023.
Net cash used in operating activities
During the years ended March 31, 2024 and 2023, our operating activities used cash of $12.3 million and $15.9 million, respectively, a decrease of $3.6 million. The reduction in cash used in operating activities during 2024 was primarily due to our May 2023 restructuring, which resulted in lower third-party research and development expenses of $4.1 million, lower salaries and benefits expenses of $2.5 million, and lower other general and administrative expenses of $0.7 million. In addition, during 2024, we earned higher interest income of $665 thousand. These amounts were offset in part in 2024 by additional cash used of $1.5 million to fund operating assets and liabilities, restructuring costs of $1.5 million, higher professional fees of $1.2 million, primarily related to the restructuring and private placement, and reduced government grants and credits of $220 thousand.
Investing activities
For the year ended March 31, 2024 our investing activities provided cash of $104 thousand compared to cash provided of $13.2 million for the year ended March 31, 2023. The decrease in cash provided was a result of a decrease in net maturities of short-term investments.
Financing activities
Net cash provided by financing activities for the year ended March 31, 2024, was $7.4 million due to the net proceeds from our September 2023 Offering of $7.3 million compared to cash provided of $304 thousand during the year ended March 31, 2023 from our at-the-market (“ATM”) program.
Private Placement
On September 24, 2023, we entered into a securities purchase agreement (the “Purchase Agreement”) with certain institutional and accredited investors in connection with a private placement offering of our securities (the “Offering”). Pursuant to the Purchase Agreement, sold 1,951,371 Class A common shares, no par value per share (the “Common Shares”), at a purchase price of $1.848 per Common Share and pre-funded warrants (the “Pre-funded Warrants”) to purchase up to 2,106,853 Common Shares at a purchase price equal to the purchase price per Common Share less $0.0001. Each Pre-funded Warrant is exercisable for one Common Share at an exercise price of $0.0001 per Common Share, was immediately exercisable, and will expire once exercised in full. Pursuant to the Purchase Agreement, we also issued to such institutional and accredited investors common warrants (the “Common Warrants”) to purchase Common Shares, exercisable for an aggregate of 2,536,391 Common Shares. Under the terms of the Purchase Agreement, for each Common Share and each Pre-funded Warrant issued in the Offering, an accompanying five-eighths (0.625) of a Common Warrant was issued to the purchaser thereof. Each whole Common Warrant is exercisable for one Common Share at an exercise price of $3.003
58
per Common Share, was immediately exercisable, and will expire on the earlier of (i) the 60th day after the date of the acceptance by the U.S. Food and Drug Administration (the “FDA”) of a New Drug Application for our product candidate GTX-104 or (ii) five years from the date of issuance. The Offering closed on September 25, 2023. The net proceeds to us from the Offering were approximately $7.3 million, after deducting fees and expenses.
At-the-Market ("ATM") Program
On June 29, 2020, we entered into an amended and restated At Market Issuance Sales Agreement (the “Sales Agreement”) with B. Riley FBR, Inc., Oppenheimer & Co. Inc. and H.C. Wainwright & Co., LLC (collectively, the “Agents”) to amend our then existing at-the-market program (the "ATM Program"). Under the terms of the Sales Agreement, which had a three-year term, we could issue and sell from time to time, common shares of the Company ("Common Shares") having an aggregate offering price of up to $75 million through the Agents. Subject to the terms and conditions of the Sales Agreement, the Agents would use their commercially reasonable efforts to sell the Common Shares from time to time, based upon our instructions. We had no obligation to sell any of the Common Shares and could, at any time, suspend sales under the Sales Agreement. We and the Agents could terminate the Sales Agreement in accordance with its terms. Under the terms of the Sales Agreement, we provided the Agents with customary indemnification rights and the Agents were entitled to compensation at a commission rate equal to 3.0% of the gross proceeds from each sale of the Common Shares. The Sales Agreement expired pursuant to its terms on June 29, 2023. We intend to examine our financing strategies on a go-forward basis and may consider entering into a new at-the-market program in the future.
During the year ended March 31, 2024, no Common Shares were sold under the ATM program. During the year ended March 31, 2023, 54,108 Common Shares were sold for total net proceeds of $304 thousand with commissions, legal expenses and costs related to the share sale amounting to $10 thousand. The Common Shares were sold at the prevailing market prices, which resulted in an average price of approximately $5.70 per share.
Contractual Obligations and Commitments
Our contractual obligations and commitments include trade payables, operating lease obligations, CMO and CRO agreements, as described below.
Research and development contracts and contract research organizations agreements
We utilize CMOs, for the development and production of clinical materials and CROs to perform services related to our clinical trials. Pursuant to the agreements with CMOs and CROs, we have either the right to terminate the agreements without penalties or under certain penalty conditions. As of March 31, 2024, we have no commitments with CMOs and $6.0 million of commitments for the next twelve months to CROs.
Raw krill oil supply contract
On October 25, 2019, we entered into a supply agreement with Aker BioMarine Antarctic AS. (“AKBM”) to purchase raw krill oil product for a committed volume of commercial starting material for CaPre, one of our former drug candidates, for a total fixed value of $3.1 million based on the value of krill oil at that time. As of March 31, 2022, the remaining balance of commitment amounted to $2.8 million. During the second calendar quarter of 2022, AKBM informed us that AKBM believed it had satisfied the terms of the supply agreement as to their obligation to deliver the remaining balance of raw krill oil product, and that we were therefore required to accept the remaining product commitment. We disagreed with AKBM’s position and believed that AKBM was not entitled to further payment under the supply agreement. Accordingly, no liability was recorded by us. The dispute remained unresolved as of both March 31, 2023 and 2022. On October 18, 2023, we entered into an agreement with AKBM to settle any and all potential claims regarding amounts due under the supply agreement (the “Settlement Agreement”). Pursuant to the terms of the Settlement Agreement, in exchange for a release and waiver of claims arising out of the supply agreement by AKBM and any of AKBM’s affiliates, we agreed to the following: (a) AKBM retained ownership of all raw krill oil product, including amounts previously delivered to us; (b) AKBM acquired and took ownership of all of our production equipment related to the production of CaPre; (c) AKBM acquired and took ownership of all of our data from research, clinical trials and pre-clinical studies with respect to CaPre; and (d) AKBM acquired and took ownership over all of our rights, title and interest in and to all intellectual property rights, including all patents and trademarks, related to CaPre owned by us. Further, AKBM acknowledged that the CaPre assets were transferred on an “as is” basis, and in connection therewith we disclaimed all representations and warranties in connection with the CaPre assets, including any representations with respect to performance or sufficiency. The value of the raw krill oil previously delivered to us, the production equipment, and the intellectual property rights related to CaPre were fully impaired in prior reporting periods and had a carrying value of nil as of March 31, 2023. As of March 31, 2024, no liability was recorded.
59
Use of Estimates and Measurement of Uncertainty
The preparation of these consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, income, and expenses. Actual results may differ from these estimates.
Estimates are based on management’s best knowledge of current events and actions that management may undertake in the future. Estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimates are revised and in any future periods affected.
Estimates and assumptions include the measurement of stock-based compensation, derivative warrant liabilities, accruals for research and development contracts and contract organization agreements, and valuation of intangibles and goodwill. Estimates and assumptions are also involved in determining which research and development expenses qualify for research and development tax credits and in what amounts. We recognize the tax credits once we have reasonable assurance that they will be realized.
Critical Accounting Policies
Valuation of Intangible Assets and Goodwill
In a business combination, the fair value of in-process research and development ("IPR&D") acquired is capitalized and accounted for as indefinite-lived intangible assets, and not amortized until the underlying project receives regulatory approval, at which point the intangible assets will be accounted for as definite-lived intangible assets or discontinued. If discontinued, the intangible assets will be written off. R&D costs incurred after the acquisition are expensed as incurred.
Our IPR&D and Goodwill was $49.3 million as of March 31, 2024, which represents 67% of total assets. Goodwill and indefinite lived assets are not amortized but are subject to an impairment review annually and more frequently when indicators of impairment exist. An impairment of goodwill could occur if the carrying amount of a reporting unit exceeds the fair value of that reporting unit. An impairment of indefinite-lived intangible assets would occur if the fair value of the intangible asset is less than the carrying value.
The nature of the assumptions in the intangible asset's impairment tests are considered critical due to a high level of subjectivity and judgment necessary to account for highly uncertain matters, and the impact of the assumptions on our financial condition and our operating performance could be material.
We test goodwill for impairment by first assessing qualitative factors to determine whether it is more likely than not that the fair value is less than its carrying amount. If we conclude it is more likely than not that fair value of the reporting unit is less than its carrying amount, a quantitative impairment test is performed. We test indefinite lived intangible assets for impairment by first assessing qualitative factors to determine whether it is more likely than not that the fair value is less than its carrying amount. Events that could result in an impairment, or trigger an interim impairment assessment, include the decision to discontinue the development of a drug, the receipt of additional clinical or nonclinical data regarding our drug candidates or a potentially competitive drug candidates, changes in the clinical development program for a drug candidate, or new information regarding potential sales for the drug candidates and increases in our weighted average cost of capital.
Individual IPR&D projects and goodwill are tested for impairment on an annual basis in the fourth quarter, and in between annual tests if an event occurs or circumstances change that would more likely than not reduce the fair value of each technology or our reporting unit below its carrying value. We identified the strategic realignment plan announced on April 4, 2023 to prioritize resources to GTX-104, from GTX-101 and GTX-102 triggered a comprehensive impairment review of our intangible assets and have considered these facts in our annual impairment test. Deferral of development for GTX-101 and 102 extended the cash runway from existing resources and also reduced the values in the discounted cashflow due to the deferral. The result of the impairment assessment resulted in the following activity between March 31, 2023 and March 31, 2024:
|
|
GTX-104 |
|
GTX-102 |
|
GTX-101 |
|
Total |
|
||||
|
|
$ |
|
$ |
|
$ |
|
$ |
|
||||
|
|
(in thousands) |
|
||||||||||
Intangible assets – in-process research and development |
|
|
|
|
|
|
|
|
|
||||
Balance, March 31, 2022 |
|
|
27,595 |
|
|
31,908 |
|
|
10,307 |
|
|
69,810 |
|
Impairment |
|
|
— |
|
|
(22,712 |
) |
|
(5,970 |
) |
|
(28,682 |
) |
Balance, March 31, 2023 |
|
|
27,595 |
|
|
9,196 |
|
|
4,337 |
|
|
41,128 |
|
Impairment |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
Balance, March 31, 2024 |
|
|
27,595 |
|
|
9,196 |
|
|
4,337 |
|
|
41,128 |
|
60
The impairment of $28.7 million of the identified intangible assets recorded for the year ended March 31, 2023 resulted in a recovery of $8.6 million of the related deferred tax liability.
|
|
$ |
|
|
|
|
(in thousands) |
|
|
Goodwill |
|
|
|
|
Balance, March 31, 2022 |
|
|
12,964 |
|
Impairment |
|
|
(4,826 |
) |
Balance, March 31, 2023 |
|
|
8,138 |
|
Impairment |
|
|
— |
|
Balance, March 31, 2024 |
|
|
8,138 |
|
The estimated fair values of our intangible assets were determined using the multi-period excess earnings method, which is a valuation methodology that provides an estimate of the fair value of an asset based on market participant expectations of the cash flows an asset would generate over its remaining useful life. The projected discounted cash flow models used to estimate the fair value of assets of our IPR&D reflect significant assumptions and are Level 3 unobservable data regarding the estimates a market participant would make in order to evaluate a drug development asset, including the following:
The projected discounted cash flow model used to estimate the fair value of our reporting unit and intangible assets as of March 31, 2024 includes a significant assumption related to each project's probability of clinical success, which is reflected in the cash flows.
The projected discounted cash flow model used to estimate the fair value of our reporting unit and the intangibles as of March 31, 2024, includes a significant assumption related to each project's projected net sales levels, which is reflected in the cash flows. Based on our fair value assessment, an impairment loss of GTX-101, GTX-102 and GTX-104 would result if the net sales assumptions decreased more than approximately 32.3%, 21.0% and 50.7% respectively, for each year, all other assumptions remaining constant. We believe that the net sales assumptions developed were applied with a conservative framework such as the exclusion of addressable markets outside the United States, which markets we expect to provide revenue upside if and when GTX-101, GTX-102 and GTX-104 are approved by the FDA.
The valuation of our IPR&D has significant measurement uncertainty given the risks and uncertainties associated with the timely and successful completion of the development and commercialization of drug candidates. We engaged a third-party valuation firm to assist us with the valuation of the IPR&D and goodwill. Assumptions are difficult to make accurately and were mainly derived from life science studies, industry data, and peer company information that our management believes represent appropriate comparable data. Estimates of value are required to be discounted to account for risks related to the inherent uncertainties of the overall development and commercialization processes.
The impairment assessment is sensitive to changes in forecasted cash flows, our selected discount rates as well as the implied control premiums. Changes to our assumptions, in particular changes in technological feasibility or changes in the regulatory approval process could materially affect the estimation of the fair value and could result in impairment charges in future quarters.
Financial Instruments
Credit Risk
Financial instruments that potentially subject the Company to a concentration of credit risk consist primarily of cash and cash equivalents. Cash and cash equivalents are all invested in accordance with the Company’s Investment Policy with the primary objective being the preservation of capital and the maintenance of liquidity, which risk is managed by dealing only with highly rated Canadian and U.S. institutions. The Company maintains its cash and cash equivalents at accredited financial institutions in amounts that exceed
61
federally insured limits. The Company does not believe that it is subject to unusual credit risk beyond the normal credit risk associated with commercial banking relationships.
Interest Rate Risk
Interest rate risk is the risk that the fair value or future cash flows of a financial instrument will fluctuate because of changes in market rates. Our exposure to interest rate risk as at March 31, 2024 was as follows:
Cash and cash equivalents |
|
Short-term fixed interest rate |
Our capacity to reinvest the short-term amounts with equivalent return will be impacted by variations in short-term fixed interest rates available on the market. Management believes the risk we will realize a loss as a result of the decline in the fair value of our short-term investments is limited because these investments have short-term maturities and are held to maturity.
Our contractual obligations related to financial instruments and other obligations and liquidity resources are presented in the liquidity and capital resources of this MD&A.
We have incurred operating losses and negative cash flows from operations in each year since our inception. We expect to incur significant expenses and continued operating losses for the foreseeable future. We expect our expenses will increase substantially in connection with our ongoing activities, particularly as we advance clinical development for our drug candidates in our pipeline; continue to engage contract manufacturing organizations to manufacture our clinical study materials and to ultimately develop large-scale manufacturing capabilities in preparation for commercial launch; seek regulatory approval for our drug candidates; and add personnel to support our drug product development and future drug product launch and commercialization.
We do not expect to generate revenue from product sales unless and until we successfully complete drug development and obtain regulatory approval, which we expect will take several years and is subject to significant uncertainty. To date, we have financed our operations primarily through public offerings and private placements of our common shares, warrants and convertible debt and with the proceeds from research tax credits. Until such time that we can generate significant revenue from drug product sales, if ever, we will require additional financing, which we expect to be sourced from a combination of public or private equity offerings or debt financing's or other non-dilutive sources, which may include fees, milestone payments and royalties from collaborations with third parties. Arrangements with collaborators or others may require us to relinquish certain rights related to our technologies or drug product candidates. Adequate additional financing may not be available to us on acceptable terms, or at all. Our inability to raise capital as and when needed could have a negative impact on our financial condition and our ability to pursue our business strategy.
We expect to have sufficient cash resources to satisfy our objectives into the second calendar quarter of 2026, which is 22 months from the issuance date of the consolidated financial statements included elsewhere in this Annual Report on Form 10-K. We require additional capital to fund our daily operating needs beyond that time. We plan to raise additional capital prior to that time in order to maintain adequate liquidity. Negative results from studies, if any, and depressed prices of our common shares could impact our ability to raise additional financing. Raising additional equity capital is subject to market conditions not within our control. If we do not raise additional funds in this time period, we may not be able to realize our assets and discharge our liabilities in the normal course of business.
Future Accounting Changes
We have considered recent accounting pronouncements and concluded that they are either not applicable to our business or that the effect is not expected to be material to our consolidated financial statements as a result of future adoption.
62
Item 7A. Quantitative and Qualitative Disclosure About Market Risk
As a smaller reporting company, we are not required to provide this information.
Item 8. Financial Statements and Supplementary Data
See our consolidated financial statements beginning on page F-1 of this Annual Report on Form 10-K.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures Disclosure Controls and Procedures
As of the end of the period covered by this Annual Report on Form 10-K, our management, with the participation of our chief executive officer (“CEO”) and principal financial officer (“PFO”), has performed an evaluation of the effectiveness of our disclosure controls and procedures within the meaning of Rules 13a-15 (e) and 15d-15(e) of the Exchange Act. Based upon this evaluation, our management has concluded that, as of March 31, 2024, our existing disclosure controls and procedures were effective. It should be noted that while the CEO and PFO believe that our disclosure controls and procedures provide a reasonable level of assurance that they are effective, they do not expect the disclosure controls and procedures to be capable of preventing all errors and fraud. A control system, no matter how well conceived or operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met.
Management’s Report on Internal Controls over Financial Reporting
Our management, with the participation of our CEO and PFO, is responsible for establishing and maintaining adequate internal control over financial reporting. Our internal control system was designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation and fair presentation of our consolidated financial statements. All internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective may not prevent or detect misstatements and can provide only reasonable assurance with respect to financial statement preparation and presentation. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. Our management conducted an assessment of the design and operation effectiveness of our internal control over financial reporting as of March 31, 2024. In making this assessment, we used the criteria established within the Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission ("COSO"). Based on this assessment, our management has concluded that, as of March 31, 2024, our internal control over financial reporting was effective.
Changes in Internal Control over Financial Reporting
No changes were made to our internal controls over financial reporting that occurred during the three months ended March 31, 2024 that have materially affected, or are reasonably likely to materially affect, our internal controls over financial reporting.
We are a non-accelerated filer under the Exchange Act and not required to comply with the auditor attestation requirements of Section 404(b) of the Sarbanes-Oxley Act of 2002. Therefore, this Annual Report on Form 10-K does not include an attestation report of our independent registered public accounting firm regarding our management’s assessment of internal control over financial reporting.
Item 9B. Other Information
During the three months ended March 31, 2024, no director or officer of the Company
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections
None.
63
PART III
Item 10. Directors, Executive Officers and Corporate Governance
The following table sets forth information with respect to our current directors and executive officers:
Name |
|
Age |
|
Position(s) held within Acasti |
|
In Office Since |
|
Current Term to Expire |
Directors |
|
|
|
|
|
|
|
|
Vimal Kavuru |
|
55 |
|
Chairman of the Board |
|
August 2021 |
|
2024 |
A. Brian Davis |
|
57 |
|
Director, Chairman of Audit Committee and Chairman of Governance and Human Resources Committee |
|
October 2023 |
|
2024 |
S. George Kottayil |
|
61 |
|
Director |
|
October 2023 |
|
2024 |
Edward Neugeboren |
|
55 |
|
Director |
|
October 2023 |
|
2024 |
Executive Officers |
|
|
|
|
|
|
|
|
Prashant Kohli |
|
52 |
|
Chief Executive Officer and Director |
|
CEO since April 2023, director since October 2023 |
|
2024 |
Robert J. DelAversano |
|
53 |
|
Vice President, Finance, Principal Financial Officer and Principal Accounting Officer |
|
January 2024 |
|
- |
Dr. R. Loch Macdonald |
|
63 |
|
Chief Medical Officer |
|
May 2023 |
|
- |
Carrie D'Andrea |
|
53 |
|
VP Clinical Operations |
|
May 2023 |
|
- |
Amresh Kumar |
|
45 |
|
VP of Program Management |
|
May 2023 |
|
- |
The following is a brief biography of our current directors and executive officers:
Vimal Kavuru
Mr. Kavuru has created and led several pharmaceutical companies. Mr. Kavuru brings, in his vision and management, a broad-based understanding of the global pharmaceutical industry with expertise in strategic planning, product and business development, and operations. In addition to previously serving as the Chairman of the Grace Therapeutics Inc. (“Grace Therapeutics”) board of directors, Mr. Kavuru is the Founder, Chairman and Chief Executive Officer of Rising Pharma Holdings, Inc., a U.S. generic pharmaceutical company, and Acetris Pharma Holdings, LLC, a generic pharmaceutical company serving U.S. government agencies, positions Mr. Kavuru has held since January 2013 and January 2016, respectively. Previously, Mr. Kavuru founded Citron Pharma and Lucid Pharma, each of which were sold to Aceto Corporation in 2016, Casper Pharma LLC, an emerging specialty brand pharmaceutical company, and Gen-Source RX, a national distributor of generic pharmaceuticals that was acquired by Cardinal Health in 2014. In 2007, Mr. Kavuru also co-founded Celon Labs, a specialty oncology and critical care pharmaceutical company that was acquired by Zanzibar Pharma Limited, a portfolio company of CDC Group. Mr. Kavuru was initially elected to the Board as a nominee of former shareholders of Grace Therapeutics in connection with Acasti’s acquisition of Grace Therapeutics. He is a registered pharmacist in the state of New York, holds a B.S. in Pharmacy from HKE College of Pharmacy, Bulgarga, India, and attended Long Island University, Brooklyn, New York with specialization in industrial pharmacy.
A. Brian Davis
Mr. Davis has nearly three decades of experience as a Chief Financial Officer and other executive financial positions in commercial and development-stage publicly traded life science companies. Mr. Davis has extensive knowledge and background related to public company accounting and financial reporting rules and regulations as well as the evaluation of financial results, internal controls and business processes. Since December 2021, Mr. Davis has been the Chief Financial Officer of XyloCor Therapeutics, Inc., a clinical-stage gene therapy company developing potential therapies for patients with cardiovascular disease. Mr. Davis was the Chief Financial Officer of Verrica Pharmaceuticals Inc., a publicly traded, NDA-stage dermatology therapeutics company, from October 2019 to July 2021. Prior to joining Verrica, Mr. Davis was the Chief Financial Officer of Strongbridge Biopharma plc, a public commercial-stage biopharmaceutical company, from March 2015 to September 2019. Mr. Davis was previously the Chief Financial Officer at Tengion, Inc., a publicly traded regenerative medicine company until Tengion, Inc. filed for bankruptcy in December 2014, and Neose Technologies, Inc., a publicly traded biopharmaceutical company. Mr. Davis is licensed as a certified public accountant, and received a B.S. in accounting from Trenton State College and an M.B.A. from The Wharton School at the University of Pennsylvania.
S. George Kottayil
64
Dr. Kottayil has over two decades of experience in the pharmaceutical industry with specific expertise in product development and drug delivery. He has several approved patents to his credit and is an inventor on multiple FDA approved drug products, a few that have achieved significant success. He co-founded two pharmaceutical drug development and drug delivery technology companies and was CEO and a member of each of their boards of directors. Most recently, from October 2014, he co-founded and was CEO and director of Grace Therapeutics, a drug delivery company with a focus on rare and orphan disease which was acquired by Acasti in August 2021. Dr. Kottayil served as Acasti’s Chief Operating Officer from September 2021 to May 2023. Dr. Kottayil has held senior positions in product development, business operations and general management at small to medium life science companies, successfully advancing drug products from bench to FDA approval and launch. He directed business operations at Unimed Pharmaceuticals Inc., a division of Solvay Pharmaceuticals, now Abbvie, from January 1993 to June 2002, and played a key role in product development and obtaining FDA approval for the company’s NDA products. Dr. Kottayil graduated with a Ph.D. in Organic and Medicinal Chemistry from the University of Kentucky.
Edward Neugeboren
Mr. Neugeboren has over three decades of healthcare experience in pharmaceutical operations, business development, corporate management, investment banking, asset management and institutional equity research. Since January of 2016, Mr. Neugeboren has served as the Chief Strategy Officer of Cronus Pharma, LLC, a fully integrated research and development, manufacturing and sales, and marketing pharmaceutical company. Mr. Neugebroren leads Cronus Pharma’s commercial operations, strategic planning and acquisitions and is also responsible for developing and executing overall corporate strategy as well as corporate and portfolio acquisitions and licensing. Previously, Mr. Neugeboren was the Chief Strategy Officer for the parent pharmaceutical group comprised of Rising Pharma Holdings, Inc., a generic pharmaceutical company and Casper Pharma, LLC, a specialty pharmaceutical company. Additionally, Mr. Neugeboren is Founder and Managing Partner of QuadView Healthcare Advisors, previously named ArcLight Advisors, LLC, a healthcare investment banking and business development firm. Mr. Neugeboren was previously a Managing Director of Ledgemont Capital Group, LLC, an investment banking firm providing strategic and financial advisory services to emerging healthcare and technology companies. Mr. Neugeboren was also a Managing Partner of Third Ridge Capital Management, LLC, a long/short U.S. equity hedge fund. Mr. Neugeboren holds Series 24, 7 and 63 FINRA security licenses and has graduated with a BA in Economics from Union College.
Prashant Kohli
Prashant Kohli has over 20 years of commercialization experience leading strategy, sales, marketing, and product management. Prior to joining Acasti in August 2021, Mr. Kohli was VP, Commercial Operations of Grace Therapeutics since December 2017. He has expertise crafting go-to-market plans for products with unique value proposition that address critical unmet needs. He has built, deployed, and led sales and marketing from the ground-up with significant experience in organization design, recruiting, performance management, incentive compensation, and P&L accountability. He has successfully implemented evidence-based, consultative-selling model that is rooted in deep understanding of the health ecosystem including patients, providers, health systems, government, and payers. He has also designed strategic marketing plans that generate leads and increase share-of-voice, augmenting the salesforce with digital tactics that increase reach and frequency. He has extensive commercial experience with specialty and small molecule drugs including in rare and orphan diseases. Prashant has worked at Archi-Tech Systems, Cardinal Health, IMS Health, Rosenbluth, and Dun & Bradstreet. He has a BA in Computer Science and Math from Augustana College and an MBA from The Wharton School.
Robert J. DelAversano
Mr. DelAversano, is a certified public accountant and has over twenty-five years of experience in accounting including thirteen years in public accounting. Mr. DelAversano joined the Company in November 2023 as Vice President, Finance. From 2018 to July 2023, Mr. DelAversano worked in roles of increasing seniority at OncoSec Medical Incorporated (“OncoSec”), a clinical-stage immuno-oncology company, which positions included Vice President of Finance, Principal Accounting Officer and Controller, and Executive Director of Finance, where he had global responsibility for accounting, external financial reporting, and financial controls covering all aspects of OncoSec’s business. Prior to joining OncoSec, Mr. DelAversano was the Director of Financial Reporting and Taxation at Brio Financial Group (“Brio”), where he served as the firm’s Director of Financial Reporting and Taxation, consulting with various public companies in financial reporting, internal control development and evaluation, budgeting and forecasting. Prior to joining Brio, Mr. DelAversano was a manager at Bartolomei Pucciarelli, LLC and oversaw its accounting and tax practice with industry focuses in manufacturing, wholesalers and medical devices services. Mr. DelAversano received a B.S. in Accounting from Rider University.
Dr. R. Loch Macdonald
Dr. Macdonald is a world-renowned practicing neurosurgeon-scientist and respected authority in subarachnoid hemorrhage. Dr. Macdonald was as Professor, Department of Surgery, Division of Neurosurgery at the University of Toronto from January 2007 until December 2019, and was Head, Division of Neurosurgery, St. Michael's Hospital, University of Toronto from January 2007 until
65
December 2015. He was Professor, Department of Neurological Surgery, Barrow Neurological Surgery, Barrow Neurological Institute, Phoenix, Arizona, from April 2018 until August 2018; Fellow, Department of Neurosurgery, University of Illinois Hospitals in Chicago, Illinois from December 2018 until June 2019; Clinical Professor, Department of Neurological Surgery, University of California San Francisco Fresno, in Fresno, California from July 2019 until September 2021; and from October 2021 to the present has been Neurosurgeon, Community Physicians Group, Community Neurosciences Institute, Community Regional Medical Center and Medical Director of Neurosciences Research, Community Health Partners. Dr Macdonald was also a founder of Edge Therapeutics, Inc. in 2009, where he was a member of the board of directors between 2009 and 2018 and was Chief Scientific Officer between 2011 and 2018. Dr. Macdonald completed his medical degree at the University of British Columbia, Vancouver, British Columbia and his PhD in Experimental Surgery at the University of Alberta in Edmonton, Alberta. He completed his Neurosurgery residency at the University of Toronto.
Carrie D’Andrea
Ms. D’Andrea is a highly experienced professional with 25 years of experience in the pharmaceutical and biotechnology industry who has built and led the planning, implementation, management, and execution of global Phase 2 and Phase 3 trials for a drug candidate for subarachnoid hemorrhage. Ms. D'Andrea was the Vice President of Clinical Operations for Edge Therapeutics Inc. from October 2014 until March 2019 and for EryDel SpA from October 2020 until April 2021. Ms. D’Andrea was a clinical operations consultant at Aegle Research from July 2021-August 2022 and Praxis Precisions Medicines from September 2022-May 2023. Ms. D’Andrea was named a Healthcare Businesswomen’s Association Rising Star in 2009 and Ms. D'Andrea received her master's degree in Pharmaceutical Quality and Regulatory Affairs from Temple University and teaches Clinical Trial Design and Operations at Rutgers University in the Master of Business and Science Program.
Amresh Kumar
Mr. Kumar is an experienced drug development, CMC, and program management expert supporting investigational and marketed products for rare diseases and neurology. Mr. Kumar is the former product leader of GTX-104 while at Grace Therapeutics (which was acquired by the Company in August 2021). Mr. Kumar acted as the Sr. Director of Program Management at Foresee Pharmaceuticals Inc. from April 2022 until May 2023 and as Program Leader and Associate Director - R&D at Grace Therapeutics between March 2015 and January 2022. Mr. Kumar received a PhD in Pharmaceutical Science from Sunrise University, India, focusing on complex injectable drug delivery systems of highly soluble oncology drugs. He has published many research articles and has more than 10 granted patents and many patent applications worldwide to his credit.
Cease Trade Orders, Bankruptcies, Penalties or Sanctions
To the knowledge of Acasti, none of our current directors or executive officers are, or have been, as at the date of this Annual Report on Form 10-K or within the 10 years prior to the date of this Annual Report on Form 10-K, a director, or executive officer of any Company (including Acasti) that:
(a) was subject to a cease trade order, an order similar to a cease trade order, or an order that denied the relevant Company access to any exemption under applicable securities legislation, that was in effect for a period of more than 30 consecutive days that was issued while the director or executive officer was acting in the capacity as director or executive officer; or
(b) was subject to an order that was issued after the director or executive officer ceased to be a director, CEO or CFO and which resulted from an event that occurred while that person was acting in the capacity as director, CEO or CFO.
To the knowledge of Acasti, other than Mr. Davis, who was previously the CFO at Tengion, Inc., a publicly traded regenerative medicine company, when it filed for bankruptcy in December 2014, none of Acasti’s current directors or executive officers:
(a) are, or have been, as at the date of this Annual Report on Form 10-K or within the 10 years prior to the date of this Annual Report on Form 10-K, a director or executive officer of any Company (including Acasti) that, while that person was acting in that capacity, or within two years of that person ceasing to act in that capacity, became bankrupt, made a proposal under any legislation relating to bankruptcy or insolvency or was subject to or instituted any proceedings, arrangement or compromise with creditors or had a receiver, receiver manager or trustee appointed to hold its assets; or
(b) have, within the 10 years prior to the date of this Proxy Statement/Prospectus, become bankrupt, made a proposal under any legislation relating to bankruptcy or insolvency, or become subject to or instituted any proceedings, arrangement or
66
compromise with creditors, or had a receiver, receiver manager or trustee appointed to hold the assets of the director or director nominee.
To the knowledge of Acasti, no current director or executive officer has been subject to:
(a) any penalties or sanctions imposed by a court relating to securities legislation or by a securities regulatory authority or has entered into a settlement agreement with a securities regulatory authority; or
(b) any other penalties or sanctions imposed by a court or regulatory body.
Family Relationships
There are no family relationships between any directors or officers of the Company.
Delinquent Section 16(a) Reports
Section 16(a) of the Exchange Act requires the Company’s directors, executive officers and greater-than-10% stockholders to file forms with the SEC to report their ownership of Company securities and any changes in ownership. We have reviewed all forms filed electronically with the SEC. Based on that review and on written information given to us by our officers and directors, we believe that all of our directors, officers and greater-than-10% stockholders filed the required reports on a timely basis under Section 16(a) during 2022, except for Mr. Macdonald, who on May 26, 2023 filed a Form 3 that was due May 18, 2023.
Code of Business Conduct and Ethics
The Board adopted a Code of Business Conduct and Ethics (“Code of Conduct”), for our directors, officers and employees on May 31, 2007, as amended from time to time. Our Code of Conduct can be found on SEDAR at www.sedar.com and on our website on www.acasti.com. A copy of the Code of Conduct can also be obtained by contacting our corporate secretary. We intend to disclose future amendments to or waivers from certain provisions of our Code of Conduct provisions on our website. Since its adoption by the Board, any breach of the Code of Conduct must be brought to the attention of the Board by our CEO or other senior executives. No report has ever been filed which pertains to any conduct of a director or executive officer that constitutes a breach to our Code of Conduct.
The Board actively monitors compliance with the Code Conduct and promotes a business environment where employees are encouraged to report malfeasance, irregularities, and other concerns. The Code of Conduct provides for specific procedures for reporting non-compliant practices in a manner which, in the opinion of the Board, encourages and promotes a culture of ethical business conduct.
The Board has also adopted a disclosure policy, insider trading policy, majority voting policy, management and board compensation policies, and a whistle blower policy.
In addition, under the Civil Code of Québec, to which we are subject as a legal person incorporated under the Business Corporations Act (Québec) (L.R.Q., c. S-31), a director must immediately disclose to the board any situation that may place him or her in a conflict of interest. Any such declaration of interest is recorded in the minutes of proceedings of the Board. In such instances, the director abstains, except if otherwise required, from the discussion and voting on the question. In addition, it is our policy that an interested director recuse himself or herself from the decision-making process pertaining to a contract or transaction in which he or she has an interest.
Audit Committee
The audit committee of the Board (the "Audit Committee") is responsible for assisting the Board in fulfilling its oversight responsibilities with respect to financial reporting, including:
67
The Audit Committee has direct communication channels with our management performing financial functions and our external auditor to discuss and review such issues as the Audit Committee may deem appropriate. The Audit Committee is composed of Mr. Davis, as Chairperson, Mr. Kavuru and Mr. Neugeboren. Each of Mr. Davis, Mr. Kavuru and Mr. Neugeboren is “financially literate” and “independent” within the meaning of the Exchange Act and applicable Nasdaq rules concerning director independence.
The Audit Committee’s charter can be found on the Company’s website at https://www.acasti.com/en/investors/corporate-governance/governance-documents.
Audit Committee Financial Expert
Our Board has determined that Mr. Davis is an “audit committee financial expert,” as defined by applicable regulations of the Securities and Exchange Commission (“SEC”). The SEC has indicated that the designation of Mr. Davis as an audit committee financial expert does not make him an “expert” for any purpose, impose any duties, obligations or liability on Mr. Davis that are greater than those imposed on members of the Audit Committee and Board who do not carry this designation, or affect the duties, obligations or liability of any other member of the Audit Committee or Board.
Item 11. Executive Compensation
Our executive compensation program is intended to attract, motivate and retain high-performing senior executives, encourage and reward superior performance, and align the executives’ interests with ours as well as those of our shareholders by providing compensation that is competitive with the compensation received by executives employed by comparable companies, and ensuring that the achievement of annual objectives is rewarded through the payment of bonuses, and providing executives with long-term incentives through the grant of stock options.
Our governance and human resources committee (“GHR”) committee, has authority to retain the services of independent compensation consultants to advise its members on executive and Board compensation and related matters, and to determine the fees and the terms and conditions of the engagement of those consultants. During our fiscal year ended March 31, 2022, the GHR committee retained compensation consulting services from FW Cook to review our executive compensation programs, including base salary, short-term and long-term incentives, total cash compensation levels and total direct compensation of certain senior positions, against those of a peer group of 20 broadly similar size, as measured by market capitalization (peer market cap all averaged less than $500M in 2021), biotechnology and pharmaceutical companies listed or headquartered in North America. The consultants also reviewed Board compensation, including advisory fees and equity incentives. All of the services provided by the consultants were provided to the GHR committee. The GHR committee assessed the independence of the consultants and concluded that its engagement of the consultants did not raise any conflict of interest with us or any of our directors or executive officers. The GHR committee continued to rely on the review of FW Cook performed during the fiscal year ended March 31, 2022 and did not retain the services of FW Cook during the fiscal year ended March 31, 2023 and, during the fiscal year ended March 31, 2024, retained the services of Pearl Meyer to review our executive compensation programs for the current fiscal year.
Compensation for our Chief Executive Officer (“CEO”) paid during the fiscal year ended March 31, 2024 was below the peer company median based on FW Cook’s review conducted during the fiscal year ended March 31, 2022.
Use of Fixed and Variable Pay Components
Compensation of our named executive officers (“NEOs"), is revised each year and has been structured to encourage and reward executive officers on the basis of short-term and long-term corporate performance. For the year ended March 31, 2024, our NEOs consisted of Prashant Kohli, our current CEO, Jan D’Alvise, our former CEO, Amresh Kumar, Vice President – Program Management, Carrie D’Andrea, Vice President – Clinical Operations, Pierre Lemieux, our former Chief Operations Officer (Canada), and Brian Ford, our former interim Chief Financial Officer.
In the context of its analysis of compensation for our fiscal year ended March 31, 2024, the following components were examined by the GHR committee:
68
For executives, more than half of their target compensation (base salary + target STIP awards + target LTIP awards) is considered “at risk.” We believe this mix results in a strong pay-for-performance relationship and alignment with shareholders and is competitive with other firms of comparable size in similar fields. The CEO (or any person acting in that capacity) makes recommendations to the GHR committee as to the compensation of our executive officers, other than the CEO, for review and approval by the Board. The GHR committee makes recommendations to the Board as to the compensation of the CEO, for approval. The CEO’s salary is based on comparable market consideration, and the GHR committee’s assessment of the CEO's performance, with regard to our financial performance, and progress in achieving key strategic business goals.
Qualitative factors beyond the quantitative financial metrics are also a key consideration in determination of individual executive compensation payments. How executives achieve their financial results and demonstrate leadership consistent with our values are key to individual compensation decisions.
Base Salary
We intend to be competitive over time with comparator companies and to attract and retain top talent. The GHR committee reviews compensation matters periodically to help ensure that it meets this strategic imperative. Base salary is set to reflect an individual’s skills, experience, and contributions within a salary structure consistent with peer group data. Base salary structure is revised annually by the GHR committee as financial and market conditions evolve.
Short Term Incentive Plan (STIP)
Our Short-Term Incentive Plan (“STIP"), provides for potential rewards when a threshold of corporate performance is met compared to the Board’s primary stated objectives for the fiscal year. Corporate performance is assessed against a table of weighted performance categories and sub-goals within each weighted category, which assessment of goal achievement funds the corporate bonus pool. These performance goals take into account the achievement of corporate milestones within timelines and budget and individual objectives determined annually by the Board according to short-term priorities. The corporate bonus pool is allocated based on achievement of personal objectives assessed through a performance grid, with pre-specified, objective performance criteria. For the most senior participants in the STIP, greater weight is assigned to corporate objectives. Target payout is expressed as a percentage of base salary, and is determined by benchmarking against peer group data. Annual salary for STIP purposes is the annual salary in effect at the end of the plan year (i.e., prior to any annual salary increases awarded for the subsequent year).
The STIP is a variable compensation plan, and all STIP payments are subject to Board approval. Participants must be employed by us at the end of the fiscal year to qualify.
Long Term Incentive Plan (LTIP)
Our Long Term Incentive Plan (“LTIP”) has been adopted as a reward and retention mechanism. Participation is determined annually at the discretion of the Board. The Acasti Pharma Inc. Stock Option Plan (the “Stock Option Plan”) is intended to align the long-term interests of participants with those of shareholders, in order to promote creation of shareholder value.
The GHR committee determines the number of stock options to be granted to a participant based on peer group data and taking into account corporate performance and the employee’s level in the organization. The LTIP calculation for NEOs is determined by both reviewing grant values and a dilution- based methodology that considers the annual grant rate as a percent of shares outstanding. All grants to NEOs during the fiscal year ended March 31, 2024, had a grant value that was below the median of the peer data prepared by FW Cook during the fiscal year ended March 31, 2022.
Our directors and executive officers are not permitted to purchase financial instruments, such as prepaid variable forward contracts, equity swaps, collars or units of exchange funds that are designed to hedge or offset a decrease in market value of equity securities granted as compensation or held, directly or indirectly, by the director or officer.
Stock Option Plan
Our Stock Option Plan was adopted by the Board on October 8, 2008, and has been amended from time to time, as most recently amended on September 28, 2022. The amendment provided for a change to the existing limits for common shares reserved for issuance under the Stock Option Plan.
The Stock Option Plan continues to provide for the granting of options to purchase common shares. The exercise price of the stock options granted under the Stock Option Plan may not be lower than the closing price of the common shares on the Nasdaq Stock Market LLC ("Nasdaq”) at the close on the day preceding the grant. The maximum number of common shares that may be issued upon exercise of options granted under the amended Stock Option Plan shall not exceed 20% of the aggregate number of issued and outstanding shares
69
of the Company as of July 28, 2022. The terms and conditions for acquiring and exercising options are set by the Board, subject among others, to the following limitations: the term of the options cannot exceed ten years and (i) all options granted to a director will be vested evenly on a monthly basis over a period of at least 12 months, and (ii) all options granted to an employee will be vested evenly on a quarterly basis over a period of at least 36 months.
The total number of shares issued to any one consultant within any twelve-month period cannot exceed 2% of the Company’s total issued and outstanding shares (on a non-diluted basis). The Company is not authorized to grant within any twelve-month period such number of options under the Stock Option Plan that could result in a number of common shares issuable pursuant to options granted to (a) related persons exceeding 2% of the Company’s issued and outstanding common shares (on a non-diluted basis) on the date an option is granted, or (b) any one eligible person in a twelve-month period exceeding 2% of the Company’s issued and outstanding common shares (on a non-diluted basis) on the date an option is granted.
The grant of options is part of the long-term incentive component of executive and director compensation and an essential part of the Company’s compensation framework. Qualified directors, employees and consultants may participate in the Stock Option Plan, which is designed to encourage option holders to link their interests with those of the Company’s shareholders, in order to promote an increase in shareholder value. As of March 31, 2024, four employees and three non-employee directors were eligible to receive awards under the Stock Option Plan. Awards and the determination of any exercise price are made by the Board, after recommendation by the GHR committee. Awards are established, among other things, according to the role and responsibilities associated with the participant’s position and his or her influence over appreciation in shareholder value. Any award grants a participant the right to purchase a certain number of common shares during a specified term in the future, after a vesting period and/or specific performance conditions, at an exercise price equal to at least 100% of the market price (as defined below) of the Company’s common shares on the grant date. The “market price” of common shares as of a particular date generally means the closing price per common share on Nasdaq. Previous awards may sometimes be taken into account when new awards are considered.
In accordance with the Stock Option Plan, all of an option holder’s options will immediately fully vest on the date of a Change of Control event (as defined in the Stock Option Plan), subject to the terms of any employment agreement or other contractual arrangement between the option holder and the Company.
However, in no case will the grant of options under the Stock Option Plan, together with any proposed or previously existing security-based compensation arrangement, result in (in each case, as determined on the grant date) the grant to any one consultant within any 12-month period, of options reserving for issuance a number of common shares exceeding in the aggregate 2% of the Company’s issued and outstanding common shares (on a non-diluted basis); or the grant to any one employee, director and/or consultant that provides investor relations services, within any 12-month period, of options reserving for issuance a number of Common shares exceeding in the aggregate 2% of the Company’s issued and outstanding common shares (on a non-diluted basis).
Options granted under the Stock Option Plan are non-transferable and are subject to a minimum vesting period of 36 months for management, and 12 months for non-executive Board members, in each case with gradual and equal vesting on no less than a quarterly basis, in the case of management, and monthly in the case of non-executive Board members. Options are exercisable, subject to vesting and/or performance conditions, at a price equal to the closing price of the common shares on Nasdaq, on the day prior to the grant of such options. In addition, and unless otherwise provided for in the relevant agreement between the Company and the holder, options will also lapse upon termination of employment or the end of the business relationship with the Company except that they may be exercised for 90 days after termination, ceasing to hold office or the end of the business relationship (30 days for investor relations services employees), in each case to the extent that they will have vested on such date of termination of employment, end of the business relationship or ceasing to hold office, as applicable, except in the case of death, disability or retirement, in which case this period is extended to 12 months.
Subject to the approval of relevant regulatory authorities, including Nasdaq, if applicable, and compliance with any conditions attached to that approval (including, in certain circumstances, approval by disinterested shareholders), if applicable, the Board has the right to amend or terminate the Stock Option Plan. However, unless option holders’ consent to the amendment or termination of the Stock Option Plan in writing, any such amendment or termination of the Stock Option Plan cannot affect the conditions of options that have already been granted and that have not been exercised under the Stock Option Plan.
As of March 31, 2024, there were 1,483,140 common shares reserved for issuance under the Stock Option Plan and Equity Incentive Plan (as defined below) and 721,793 options outstanding under the Stock Option Plan.
Equity Incentive Plan
On May 22, 2013, the Acasti Pharma Inc. Equity Incentive Plan (the “Equity Incentive Plan”) was adopted by the Board in order to, among other things, provide us with a share-related mechanism to attract, retain and motivate qualified directors, employees and consultants. The adoption of the Equity Incentive Plan was initially approved by shareholders on June 27, 2013, and has been amended from time to time, as most recently amended on August 4, 2022, and approved by shareholders on September 28, 2022.
70
Eligible persons may participate in the Equity Incentive Plan. “Eligible persons” under the Equity Incentive Plan consist of any director, officer, employee, or consultant (as defined in the Equity Incentive Plan) of our Company or a subsidiary. A participant is an eligible person to whom an award has been granted under the Equity Incentive Plan. The Equity Incentive Plan provides us with the option to grant to eligible persons bonus shares, restricted shares, restricted share units, performance share units, deferred share units and other share-based awards.
The Board has the discretion to determine that any unvested or unearned restricted share units, deferred share units, performance share units or other share-based awards or restricted shares subject to a restricted period outstanding immediately prior to the occurrence of a change in control will become fully vested or earned or free of restriction upon the occurrence of a change in control. The Board may also determine that any vested or earned restricted share units, deferred share units, performance share units or other share-based awards will be cashed out based on the market price of our common shares as of the date a change in control is deemed to have occurred, or as of such other date as the Board may determine prior to the change in control. Further, the Board has the right to provide for the conversion or exchange of any restricted share unit, deferred share unit, performance share unit or other share-based award into or for rights or other securities in any entity participating in or resulting from the change in control.
The Equity Incentive Plan is administered by the Board and the Board has sole and complete authority, in its discretion, to determine the type of awards under the Equity Incentive Plan relating to the issuance of common shares (including any combination of bonus shares, restricted share units, performance share units, deferred share units, restricted shares or other share-based awards) in such amounts, to such persons and under such terms and conditions as the Board may determine, in accordance with the provisions of the Equity Incentive Plan and the recommendations made by the GHR committee.
Subject to the adjustment provisions provided for in the Equity Incentive Plan and the applicable rules and regulations of all regulatory authorities to which we are subject (including any stock exchange), the total number of common shares reserved for issuance pursuant to awards granted under the Equity Incentive Plan will be equal to a number that will not exceed 20% of the issued and outstanding common shares as of July 28, 2022, which number shall include common shares issuable pursuant to options issued under the stock option plan.
As of March 31, 2024, there were 1,483,140 common shares reserved for issuance under the Equity Incentive Plan and Stock Option Plan and no awards outstanding under the Equity Incentive Plan.
Other Forms of Compensation
Retirement Plans. We sponsor a voluntary Registered Retirement Savings Plan ("RRSP"), matching program, which is open to all eligible employees, including NEOs, who reside in Canada. The RRSP matching program matches employees’ contributions up to a maximum of $1,500 per fiscal year for eligible employees who participate in the program. We currently have no eligible employees who reside in Canada. We have also implemented a 401K plan for US employees. Because of the size of our current employee population in the US and to assure passage of anti-discrimination testing, the 401K plan has a “safe harbor” provision which provides a contribution of 3% of salary to the 401K accounts of all eligible US employees, including NEOs who reside in the US.
Other Benefits and Perquisites. Our executive employee benefit program also includes life, medical, dental and disability insurance. These benefits and perquisites are designed to be competitive overall with equivalent positions in comparable organizations. We do not have a pension plan for employees.
Compensation Governance
Compensation of our executive officers and directors is recommended to the Board by the GHR committee. In its review process, the GHR committee informally reviews executive and corporate performance on a quarterly basis, with input from management. Annually, the GHR committee conducts a more formal review and assessment of executive and corporate performance. The GHR committee is composed of the following members: Mr. Kavuru (Chairman), Mr. Davis and Mr. Neugeboren, each of whom is independent within the meaning of applicable Nasdaq rules. The GHR committee establishes management compensation policies and oversees their general implementation. All members of the GHR committee have direct experience which is relevant to their responsibilities as GHR committee members. All GHR committee members are or have held senior executive or director roles within significant businesses in our industry, some also having public companies experience, and have a level of financial understanding which allows them to assess the costs versus benefits of compensation plans. The GHR committee’s members’ combined experience in our sector provides them with a good understanding of our success factors and risks, which are highly relevant to determining metrics for measuring success.
We do not believe that our compensation program results in unnecessary or inappropriate risk taking, including risks that are likely to have a material adverse effect on us. Payments of bonuses, if any, are not made unless performance goals are met.
2024 Summary Compensation Table
71
The following table sets forth the compensation information for NEOs, which includes all persons who served as our principal executive officer during the fiscal year ended March 31, 2024, our two most highly compensated executive officers other than our principal executive officer, who were serving as executive officers as of March 31, 2024, and two individuals who would have been our most highly compensated executive officers but for the fact that they were no longer serving as executive officers as of March 31, 2024.
Name and |
|
Year |
Salary ($) |
|
Bonus ($) |
|
Option Awards ($)(1) (2) |
|
All Other Compensation ($)(3) |
|
Total Compensation ($) |
|
||||||
Jan D'Alvise (4) |
|
March 31, 2024 |
|
$ |
51,577 |
|
$ |
- |
|
$ |
- |
|
$ |
579,450 |
|
$ |
631,027 |
|
Former President and CEO |
|
March 31, 2023 |
|
$ |
494,761 |
|
$ |
210,642 |
|
$ |
426,799 |
|
$ |
- |
|
$ |
1,132,202 |
|
Prashant Kohli (5) |
|
March 31, 2024 |
|
$ |
399,970 |
|
$ |
200,000 |
|
$ |
387,660 |
|
$ |
- |
|
$ |
987,630 |
|
CEO |
|
March 31, 2023 |
|
$ |
379,370 |
|
$ |
75,270 |
|
$ |
57,103 |
|
$ |
- |
|
$ |
511,743 |
|
Amresh Kumar (6) |
|
March 31, 2024 |
|
$ |
243,269 |
|
$ |
82,500 |
|
$ |
52,250 |
|
$ |
- |
|
$ |
378,019 |
|
VP Program Management |
|
March 31, 2023 |
|
$ |
- |
|
$ |
- |
|
$ |
- |
|
$ |
- |
|
$ |
- |
|
Carrie D'Andrea (7) |
|
March 31, 2024 |
|
$ |
248,250 |
|
$ |
82,500 |
|
$ |
52,250 |
|
$ |
- |
|
$ |
383,000 |
|
VP Clinical Operations |
|
March 31, 2023 |
|
$ |
- |
|
$ |
- |
|
$ |
- |
|
$ |
- |
|
$ |
- |
|
Pierre Lemieux (8) |
|
March 31, 2024 |
|
$ |
84,558 |
|
$ |
- |
|
$ |
- |
|
$ |
347,316 |
|
$ |
431,874 |
|
Former COO, Canada and CSO |
|
March 31, 2023 |
|
$ |
304,450 |
|
$ |
111,585 |
|
$ |
125,753 |
|
$ |
- |
|
$ |
541,788 |
|
Brian Ford (9) |
|
March 31, 2024 |
|
$ |
177,689 |
|
$ |
- |
|
$ |
116,588 |
|
$ |
227,203 |
|
$ |
521,480 |
|
Former Interim CFO |
|
March 31, 2023 |
|
$ |
278,825 |
|
$ |
100,522 |
|
$ |
125,753 |
|
$ |
- |
|
$ |
505,100 |
|
Outstanding Equity Awards at March 31, 2024
The following tables provide information about the number and value of the outstanding option-based awards held by the NEOs as of March 31, 2024
72
|
|
Option awards |
||||||||||||||||
|
|
Number of securities underlying unexercised options (#) |
|
|
Number of securities underlying |
|
|
Equity incentive |
|
|
Option exercise |
|
|
Option expiration date |
||||
Jan D'Alvise, Former President and CEO |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
$ |
— |
|
|
|
Prashant Kohli, CEO |
|
|
15,515 |
|
|
|
5,169 |
|
|
|
— |
|
|
$ |
9.90 |
|
|
November 12, 2031 |
|
|
|
7,294 |
|
|
|
5,206 |
|
|
|
— |
|
|
$ |
5.34 |
|
|
June 22, 2032 |
|
|
|
52,084 |
|
|
|
156,250 |
|
|
|
— |
|
|
$ |
2.64 |
|
|
July 14, 2033 |
|
|
|
41,668 |
|
|
|
— |
|
|
|
— |
|
|
$ |
2.13 |
|
|
December 19, 2033 |
Amresh Kumar, VP Program Management |
|
|
10,500 |
|
|
|
31,500 |
|
|
|
— |
|
|
$ |
2.64 |
|
|
July 14, 2033 |
Carrie D'Andrea, VP Clinical Operations |
|
|
10,500 |
|
|
|
31,500 |
|
|
|
— |
|
|
$ |
2.64 |
|
|
July 14, 2033 |
Pierre Lemieux, Former COO, Canada |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
$ |
— |
|
|
|
Brian Ford, Former Interim CFO |
|
|
35,990 |
|
|
|
11,994 |
|
|
|
— |
|
|
$ |
9.90 |
|
|
November 12, 2031 |
|
|
|
16,044 |
|
|
|
11,456 |
|
|
|
— |
|
|
$ |
5.34 |
|
|
June 22, 2032 |
The option awards and exercise prices listed above have been adjusted to account for the Reverse Stock Split. The option awards listed in the table above vest with respect to 1/12 on each quarterly anniversary thereafter over the following three years, subject to the executive officer’s continuous service with the Company through the vesting date. The option awards listed above will be cancelled 90 days after termination date, as per the Stock Option Plan.
Employment Agreements with Named Executive Officers
Jan D’Alvise, Former President and CEO
On June 1, 2016, the Company entered into an executive employment agreement with Ms. D’Alvise. Pursuant to her executive employment agreement, Ms. D’Alvise’s annual base salary was set at $330,000 and she was eligible to receive annual performance bonuses based on a target amount of 50% of her annual base salary with a maximum of up to 80% of her annual base salary. In accordance with the terms and provisions of the executive employment agreement the Company entered into with Ms. D’Alvise, the Company had the right to terminate the executive’s employment at any time for “good and sufficient cause”, as defined in the employment agreement, without notice or severance. The Company had the right to terminate the executive’s employment at any time without cause or upon a Change of Control, as defined in the Stock Option Plan, by providing the executive with sixty days’ notice of termination and payment equal to twelve months’ base salary plus any bonus payable. The executive was able to resign from employment upon providing the Company with at least sixty days’ advance written notice. The executive was able to terminate employment with “good reason”, as defined in the executive employment agreement, in which case the Company would be required to make payment equal to twelve months’ base salary plus any bonus payable. Ms. D’Alvise ceased to be the Company’s President and CEO effective April 4, 2023.
Prashant Kohli, Chief Executive Officer
Mr. Kohli became Chief Executive Officer of Acasti on April 4, 2023. Pursuant to his employment arrangement, Mr. Kohli’s annual base salary is set at $400,000 and he is eligible to receive annual performance bonuses of up to 50% of his annual base salary. In accordance with the terms of Mr. Kohli’s employment arrangement, his employment could be terminated by the Company or by Mr. Kohli at any time with or without cause.
Amresh Kumar, VP Program Management
On May 8, 2023 we entered into a employee agreement with Amresh Kumar, pursuant to which he is entitled to an annual salary of $275,000 and he is eligible to receive annual performance bonuses of up to 30% of his annual base salary.
Carrie D’Andrea, VP Clinical Operations
73
On May 8, 2023 we entered into a consulting agreement with Carrie D'Andrea (“Ms. D’Andrea's Consulting Agreement”). Ms. D’Andrea’s Consulting Agreement provides, among other things, that Ms. D'Andrea will serve as a non-employee vice-president of clinical operations on a full-time basis, in exchange for a fee of $18,000 per month. There is no arrangement or understanding between Ms. D’Andrea and any other persons pursuant to which Ms. D’Andrea was selected as an officer. On July 1, 2023, we entered into an employment agreement with Ms. D'Andrea, pursuant to which she is entitled to an annual salary of $275,000 and she is eligible to receive annual performance bonuses of up to 30% of her annual base salary.
Pierre Lemieux, Former COO (Canada)
On September 26, 2017, the Company entered into an executive employment agreement with Dr. Pierre Lemieux. Pursuant to his executive employment agreement, Dr. Lemieux’s annual base salary was set at CAD$253,700 and he was eligible to receive annual performance bonuses of up to 40% of his annual base salary. In accordance with the terms and provisions of Dr. Lemieux’s executive employment agreement, the Company had the right to terminate the executive’s employment at any time for “good and sufficient cause”, as defined in the employment agreement, without notice or severance. The Company had the right to terminate the executive’s employment at any time without cause or upon a Change of Control, as defined in the Stock Option Plan, by providing the executive with thirty days’ notice of termination and payment equal to twelve months’ base salary plus any bonus payable. The executive was entitled to resign from employment upon providing us with at least sixty days' advance written notice. The executive was able to resign from employment upon providing the Company with at least sixty days’ advance written notice. The executive was able to terminate employment with “good reason”, as defined in the executive employment agreement, in which case the Company would be required to make payment equal to twelve months’ base salary. Dr. Lemieux ceased to be the Company's Chief Operating Officer (Canada) effective May 8, 2023. Dr. Lemieux received total payments of $431,874 upon termination, that included a one-time separation payment of $347,316.
Brian Ford, Interim CFO
On September 13, 2021, we entered into an executive employment agreement with Mr. Ford. Pursuant to his executive employment agreement, Mr. Ford’s annual base salary was set at CAD$350,000 and he was eligible to receive annual performance bonuses of up to 40% of his annual base salary. In accordance with the terms and provisions of the executive employment agreement we entered into with Mr. Ford, we were entitled to terminate his employment at any time with cause. We were entitled to terminate the executive’s employment without cause by providing the executive employee, with either a payment equal to six months of base salary, plus two months of base salary for each completed year of service, to a maximum of twelve months in total, or a payment equal to twelve months of base salary in the event that such a termination occurs within three months following a change of control, as defined in our stock option plan. The executive was entitled to resign from employment and upon providing us with at least eight weeks of advance written notice. Effective May 8, 2023, Mr. Ford employment as the Company’s Chief Financial Officer was terminated and he is entitled to severance payment in accordance with the terms of his executive employment agreement.
Clawback Policy
We have a compensation recoupment, or clawback, policy, which we adopted to comply with Nasdaq listing standards implementing Exchange Act Rule 10D-1. The clawback policy includes mandatory recoupment of excess incentive-based compensation received by a covered executive (including the Named Executive Officers) on or after October 2, 2023 in the event of a restatement of the Company’s consolidated financial statements due to material non-compliance with any financial reporting requirement under federal securities laws, as required by Exchange Act Rule 10D-1.
Non-Executive Director Compensation
Our non-executive directors’ compensation consists of an annual fixed compensation of $60,000 for the chairman of the Board and $35,000 for the other non-executive Board members. In addition, the chairperson of the Audit Committee and the chairperson of the GHR committee receive additional compensation of $15,000 and $12,000, respectively, while members of the Audit Committee and the GHR committee receive additional compensation of $7,500 and $6,000, respectively. The directors are also entitled to a fee of $1,000 per non-regularly scheduled Board meeting as well as a reimbursement for traveling and other reasonable expenses properly incurred by them in attending meetings of the Board or any committee or in otherwise serving us, in accordance with our policy on travel and expenses.
Following their first election to our Board, non-executive directors are eligible to receive an initial equity grant of up to 150% of their annual cash retainer worth of stock options vesting monthly in equal installments over a 12-month period, subject to the other terms and conditions set forth under the heading “Stock Option Plan.” In addition to their initial grant, non-executive directors are eligible to receive an annual equity-based award equal to 100% of their total annual cash retainer vesting monthly in equal installments over a 12-month period. These awards will be granted at the same time that we are performing our annual performance review for our employees, subject to availability of common shares and subject to the terms and conditions described under the headings “Stock Option Plan” and
74
“Equity Incentive Plan.” The level of these awards are intended to be consistent with equivalent awards by comparable companies obtained from our benchmarking exercise and in accordance with the recommendations obtained from our independent compensation consultant.
The following table sets forth compensation for each non-executive director for the fiscal year ended March 31, 2024. Mr. Kohli does not receive any additional compensation for his service as a director. Information regarding the compensation for Mr. Kohli is reflected in the “2024 Summary Compensation Table” set forth above.
The total compensation for our non-executive directors during fiscal year ended March 31, 2024, was as follows:
Name |
|
Fees earned or paid in cash |
|
|
Stock awards |
|
|
Option awards |
|
|
Non-equity incentive plan compensation |
|
|
Nonqualified deferred compensation earnings |
|
|
All other compensation |
|
|
Total |
|
|||||||
Vimal Kavuru (2) |
|
|
85,100 |
|
|
|
— |
|
|
|
42,332 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
127,432 |
|
Donald Olds (3) |
|
|
30,400 |
|
|
|
— |
|
|
|
29,972 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
60,372 |
|
Michael Derby (3) |
|
|
25,400 |
|
|
|
— |
|
|
|
33,378 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
58,778 |
|
Brian Davis (4) |
|
|
33,600 |
|
|
|
— |
|
|
|
23,897 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
57,497 |
|
George Kottayil (4) |
|
|
21,000 |
|
|
|
— |
|
|
|
23,897 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
44,897 |
|
Edward Neugeboren (4) |
|
|
29,100 |
|
|
|
— |
|
|
|
23,897 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
52,997 |
|
Item 402(v) Pay Versus Performance
The disclosure included in this section is prescribed by SEC rules and does not necessarily align with how the Company or the GHR committee view the link between the Company’s performance and named executive officer pay. This disclosure is intended to comply with the requirements of Item 402(v) of Regulation S-K applicable to “smaller reporting companies.”
Required Tabular Disclosure of Pay Versus Performance
As required by Section 953(a) of the Dodd-Frank Wall Street Reform and Consumer Protection Act and Item 402(v) of Regulation S-K, we are providing the following information about the relationship between executive compensation actually paid and certain financial performance of the Company. The following table sets forth information concerning Compensation Actually Paid (“CAP”) to our Principal Executive Officers (“PEO”) and non-PEO NEOs versus our total shareholder return (“TSR”) and net income (loss) performance results for the fiscal years ended March 31, 2024, 2023, and 2022. The amounts set forth below under the headings “Compensation Actually Paid to PEO” and “Average Compensation Actually Paid to Non-PEO NEOs” have been calculated in a manner consistent with Item 402(v) of Regulation S-K. Use of the term CAP is required by the SEC’s rules and as a result of the calculation methodology required by the SEC, such amounts differ from compensation actually received by the individuals and the compensation decisions described in the “Executive Compensation Summary” section above.
The 2023 CAP to our PEO and the average CAP to our non-PEO NEOs reflects the following adjustments required by the applicable SEC rules from the total compensation reported in the Summary Compensation Table (“SCT”):
75
Year |
|
Summary compensation table total for PEO 1 ($) |
|
|
Compensation actually paid to PEO 1 ($) |
|
|
|
Summary compensation table total for PEO 2 ($) |
|
|
Compensation actually paid to PEO 2 ($) |
|
|
Average summary compensation table total for non-PEO NEOs ($) |
|
|
Average compensation actually paid to non-PEO NEOs ($) |
|
|
Value of initial fixed $100 investment based on: Total shareholder return (TSR) ($) |
|
|
Net income (loss) ($ in 000s) |
|
||||||||
(a) |
|
(b) |
|
|
(c) |
|
|
|
(d) |
|
|
(e) |
|
|
(f) |
|
|
(g) |
|
|
(h) |
|
|
(i) |
|
||||||||
March 31, 2024 |
|
|
631,027 |
|
|
|
631,027 |
|
|
|
|
987,630 |
|
|
|
1,224,297 |
|
|
|
428,593 |
|
|
|
718,671 |
|
|
|
12.11 |
|
|
|
(12,853 |
) |
March 31, 2023 |
|
|
1,132,202 |
|
|
|
705,403 |
|
|
|
|
511,743 |
|
|
|
454,640 |
|
|
|
504,564 |
|
|
|
596,611 |
|
|
|
9.32 |
|
|
|
(42,429 |
) |
March 31, 2022 |
|
|
1,546,663 |
|
|
|
445,161 |
|
|
|
|
655,256 |
|
|
|
141,000 |
|
|
|
729,703 |
|
|
|
766,415 |
|
|
|
26.27 |
|
|
|
(9,819 |
) |
(1) Our Total Shareholder Return (“TSR”) for each of the applicable fiscal years is calculated based on a fixed investment of $100 at the applicable measurement point (March 31, 2021) on the same cumulative basis as is used in Item 201(e) of Regulation S-K.
(2) Net loss is as reported in our consolidated financial statements.
The 2024 CAP to our PEOs and the average CAP to our non-PEO NEOs reflects the following adjustments required by the applicable SEC rules from the total compensation reported in the SCT:
|
|
PEO 1 |
|
|
PEO 2 |
|
|
Average of Non-PEO NEOs |
|
|||
Total Reported in 2024 SCT |
|
$ |
631,027 |
|
|
$ |
987,630 |
|
|
$ |
428,593 |
|
Less: value of equity award reported in the SCT |
|
$ |
— |
|
|
$ |
(387,660 |
) |
|
$ |
(221,088 |
) |
Add: year-end value of equity awards granted in 2024 that are unvested and outstanding |
|
$ |
— |
|
|
$ |
201,041 |
|
|
$ |
120,473 |
|
Add: change in fair value (from prior year-end) of prior year equity awards that are unvested and outstanding |
|
$ |
— |
|
|
$ |
23,482 |
|
|
$ |
140,187 |
|
Add: fair market value of equity awards granted in 2024 and that vested in 2024 |
|
$ |
— |
|
|
$ |
229,097 |
|
|
$ |
127,068 |
|
Add: change in fair value (from prior year-end) of prior year equity awards that vested in 2024 |
|
$ |
— |
|
|
$ |
170,707 |
|
|
$ |
123,438 |
|
Compensation Actually Paid for 2024 |
|
$ |
631,027 |
|
|
$ |
1,224,297 |
|
|
$ |
718,671 |
|
In accordance with Item 402(v) of Regulation S-K, we are providing the following descriptions of the relationships between information presented in the Pay Versus Performance table above.
Compensation Actually Paid and Net Income (Loss)
Due to the nature of our consolidated financial statements and primary focus on research and development of novel therapies, we have not historically utilized net income (loss) as a performance measure for our executive compensation program. As a result, we do not believe there is any meaningful relationship between our net loss and compensation actually paid to our NEOs during the periods presented.
Compensation Actually Paid and TSR
We do not utilize TSR in our executive compensation program. However, we do utilize several other performance measures to align executive compensation with performance. As described in more detail above, part of the compensation NEOs are eligible to receive consists of annual performance-based cash bonuses that are designed to provide appropriate incentives to the Company’s executives to achieve defined annual corporate goals and to reward executives for individual achievement towards these goals, subject to certain employment criteria. Additionally, the Board views stock options, which are an integral part of our executive compensation program, as related to company performance although not directly tied to TSR, because they provide value only if the market price of our common shares increase, and if the executive officer continues in the Company’s employment over the vesting period. These stock option awards align the Company’s executive officers’ interests with those of its shareholders by providing a continuing financial incentive to maximize long-term value for Shareholders and by encouraging the Company’s executive officers to continue in employment for the long-term.
76
The following graphs sets forth the relationship between CAP to our PEO 1, CAP to our PEO 2, the average of CAP to our Non-PEO NEOs, and the Company's net loss TSR over the period covering 2024, 2023, and 2022.
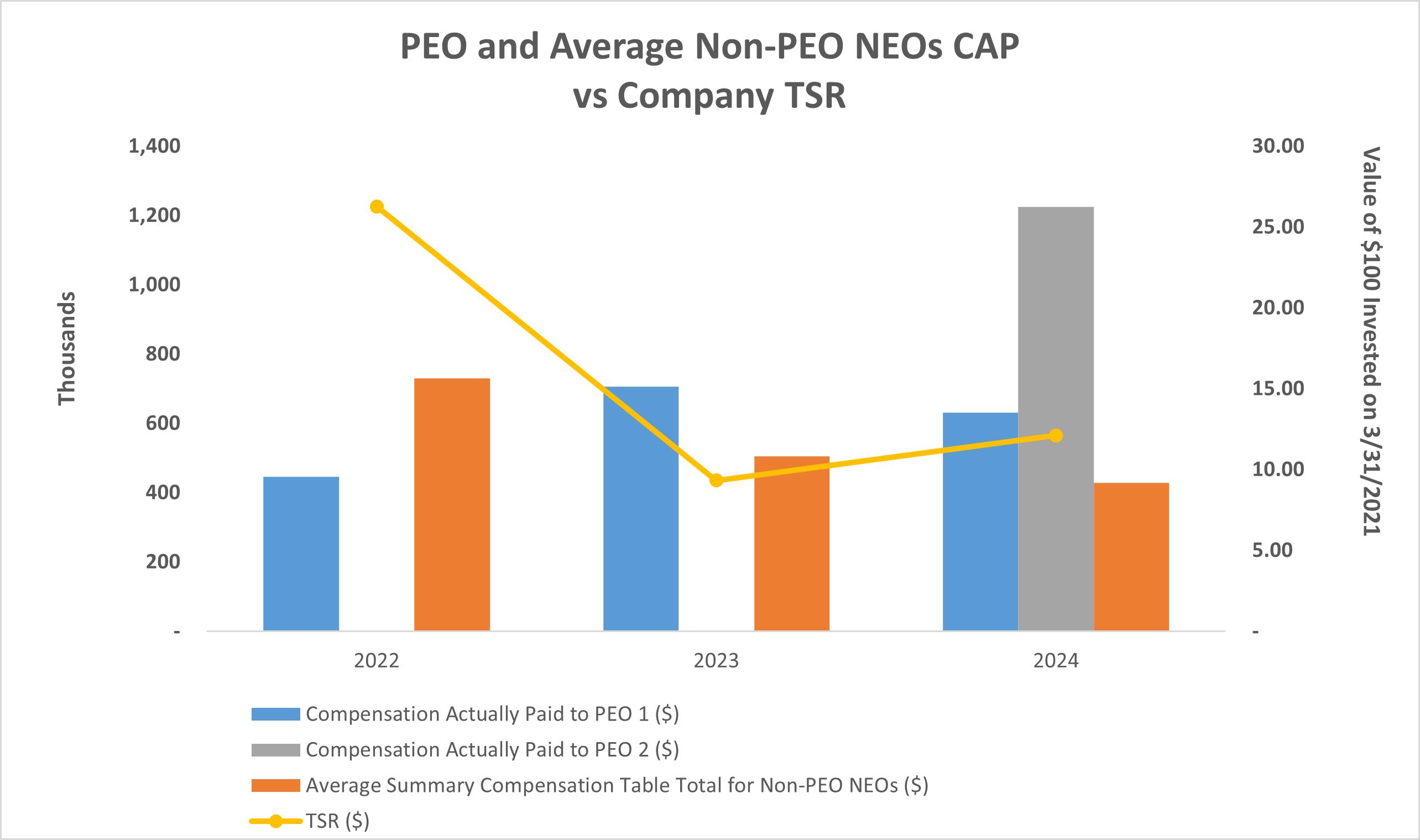
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Shareholder Matters Equity Compensation Plan Information
77
The following table sets forth certain information regarding the Company’s equity compensation plans as of March 31, 2024:
|
|
(a) Number of |
|
|
(b) Weighted- |
|
|
(c) Number of |
|
|||
Equity compensation plans approved by security holders (Stock Option Plan)(1): |
|
|
721,793 |
|
|
$ |
3.68 |
|
|
|
761,347 |
|
Equity compensation plans approved by security holders (Equity Incentive Plan)(2): |
|
|
— |
|
|
$ |
— |
|
|
|
— |
|
Equity compensation plans not approved by security holders: |
|
|
— |
|
|
$ |
— |
|
|
|
— |
|
Total |
|
|
721,793 |
|
|
$ |
3.68 |
|
|
|
761,347 |
|
Notes:
Security Ownership of Certain Beneficial Owners
The following table sets forth certain information regarding beneficial ownership of our common shares as of May 31, 2024 by each director and the named executive officers identified above, and all directors and executive officers as a group. Beneficial ownership is
78
determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. All common shares have the same voting rights.
For the purposes of calculating percentage ownership, as of May 31, 2024, 9,399,404 common shares were issued and outstanding, and, for any individual who beneficially owns common shares represented by options exercisable within 60 days of May 31, 2024, these shares are treated as if outstanding for that person, but not for any other person.
Name and Address |
|
Amount and Nature |
|
|
Percentage of |
|
||
Prashant Kohli (2) |
|
|
168,775 |
|
|
1.40% |
|
|
Amresh Kumar (3) |
|
|
26,763 |
|
|
* |
|
|
Carrie D'Andrea (4) |
|
|
15,645 |
|
|
* |
|
|
Brian Davis (5) |
|
|
12,430 |
|
|
* |
|
|
Vimal Kavuru (6) |
|
|
460,215 |
|
|
4.88% |
|
|
George Kottayil (7) |
|
|
507,128 |
|
|
5.39% |
|
|
Edward Neugeboren (8) |
|
|
50,325 |
|
|
* |
|
|
Brian Ford (9) |
|
|
58,324 |
|
|
* |
|
|
Jan D'Alvise |
|
|
— |
|
|
|
— |
|
Pierre Lemieux |
|
|
— |
|
|
|
— |
|
SS Pharma LLC (10) |
|
|
931,743 |
|
|
9.91% |
|
|
Shore Pharma LLC (11) |
|
|
1,188,076 |
|
|
12.64% |
|
|
AIGH Capital Management, LLC, AIGH Investment Partners LLC, and Orin Hirschman (12) |
|
|
567,812 |
|
|
6.04% |
|
|
Bank of America Corporation (13) |
|
|
494,698 |
|
|
5.26% |
|
|
Rajitha Grace 2018 Irrevocable Trust (14) |
|
|
781,592 |
|
|
8.32% |
|
|
Directors and officers as a group (9 persons) |
|
|
1,270,771 |
|
|
13.13% |
|
|
* Less than 1%.
Notes:
79
Changes in Control
There existed no change in control arrangements at March 31, 2024
Item 13. Certain Relationships and Related Transactions and Director Independence.
Related Party Transaction
As set forth in its charter, the Audit Committee is tasked with the review and approval of any proposed transactions with related persons of the Company. After initial review and approval of any proposed transaction with a related person, the Audit Committee continues to oversee and review any such transactions on a quarterly basis to ensure that such transaction continues to fall within the parameters of such initial approval.
Other than as set forth below, since April 1, 2022, there were no transactions or any currently proposed transactions in which the Company was or is to be a participant and the amounts exceeds $120,000, and in which any related person had or will have a direct or indirect interest.
Private Placement Offering
On September 24, 2023, we entered into a securities purchase agreement (the “Purchase Agreement”) with certain institutional and accredited investors in connection with a private placement offering of our securities (the “Offering”). Pursuant to the Purchase Agreement, sold 1,951,371 Class A common shares, no par value per share (the “Common Shares”), at a purchase price of $1.848 per Common Share and pre-funded warrants (the “Pre-funded Warrants”) to purchase up to 2,106,853 Common Shares at a purchase price equal to the purchase price per Common Share less $0.0001. Each Pre-funded Warrant is exercisable for one Common Share at an exercise price of $0.0001 per Common Share, was immediately exercisable, and will expire once exercised in full. Pursuant to the Purchase Agreement, we also issued to such institutional and accredited investors common warrants (the “Common Warrants”) to purchase Common Shares, exercisable for an aggregate of 2,536,391 Common Shares. Under the terms of the Purchase Agreement, for each Common Share and each Pre-funded Warrant issued in the Offering, an accompanying five-eighths (0.625) of a Common Warrant was issued to the purchaser thereof. Each whole Common Warrant is exercisable for one Common Share at an exercise price of $3.003 per Common Share, was immediately exercisable, and will expire on the earlier of (i) the 60th day after the date of the acceptance by the U.S. Food and Drug Administration (the “FDA”) of a New Drug Application for our product candidate GTX-104 or (ii) five years from the date of issuance.
The Offering closed on September 25, 2023. The net proceeds to us from the Offering were approximately $7.3 million, after deducting fees and expenses.
Shore Pharma LLC, an entity controlled by Vimal Kavuru, the Chair of our Board, and SS Pharma LLC, the beneficial owners of 6.9% and 5.5%, respectively, of the Common Shares prior to the Offering, each a related party of Acasti, participated in the Offering. Each of Shore Pharma LLC and SS Pharma LLC purchased $1,250,000 of securities from us in the Offering.
Director Independence
Our board of directors (the "Board") believes that, in order to maximize its effectiveness, the Board must be able to operate independently. A majority of directors must satisfy the applicable tests of independence set forth in the Nasdaq rules and promulgated by the SEC, such that the Board complies with all independence requirements under applicable corporate and securities laws and stock exchange requirements applicable to us. No director will be independent unless the Board has affirmatively determined that the director has no material relationship with us or any of our affiliates, either directly or indirectly or as a partner, shareholder or officer of an organization that has a relationship with us or our affiliates. Such determinations will be made on an annual basis and, if a director joins the board of directors between annual meetings, at such time.
Independent Directors
80
The Board determined that Mr. Kavuru, Mr. Davis and Mr. Neugeboren are independent within the meaning of the Nasdaq rules. In making its independence determination, the Board considered Mr. Kavuru’s participation in the Offering, as described above.
Chairman of the Board
Mr. Kavuru acts as chairman of the Board. His duties and responsibilities consist of the oversight of the quality and integrity of the Board’ practices.
Board Mandate
The Board is responsible for overseeing management in carrying out the business and affairs of the Company. Directors are required to act and exercise their powers with reasonable prudence in the best interests of the Company. The Board agrees with and confirms its responsibility for overseeing management's performance in the following particular areas:
In carrying out its mandate, the Board relies primarily on management to provide it with regular detailed reports on the operations of the Company and its financial position. The Board reviews and assesses these reports and other information provided to it at meetings of the Board and/or of its committees. At least annually, the Board approves a strategic plan for the Company, taking into account, among other things, the opportunities and risks of the Company’s business, its risk appetite, emerging trends, and the competitive environment in the industry.
Position Descriptions
A written position description has been approved for the chairs of each committee of the Board. The primary role and responsibility of the chair of each committee of the Board is to: (i) in general, ensure that the committee fulfills its mandate, as determined by the Board and in accordance with the committee’s charter; (ii) chair meetings of the committee; (iii) report to the Board; and (iv) act as liaison between the committee and the Board and our management.
The Board has adopted a written position description for the chairman of the Board. The chairman of the Board is responsible for leading the board to fulfill its duties under the Board’s mandate as independent of management and acting as an advisor to the chief executive officer. The chairman’s duties include, but are not limited to, setting meeting agendas, approving and supervising management’s progress towards achieving strategic goals, chairing meetings and working with the respective committee and management to ensure, to the greatest extent possible, the effective functioning of the committee and the Board. The chairman must oversee that the relationship between the Board, management of the Company, the Company’s shareholders and other stakeholders are effective, efficient, and further to the best interests of the Company.
Orientation and Continuing Education
We provide orientation for new appointees to the Board and committees in the form of informal meetings with members of the Board and senior management, complemented by presentations on the main areas of our business. The Board does not formally provide continuing education to its directors, as directors are experienced members. The Board relies on third-party professional assistance, when judged necessary, in order to be educated/updated on a particular topic.
Nomination of Directors
The Board receives recommendations from the GHR committee, but retains responsibility for managing its own affairs by, among other things, giving its approval for the composition and size of the Board, and the selection of candidates nominated for election to the Board.
81
The GHR committee initially evaluates candidates for nomination for election as directors, having regard to the background, diversity, employment, and qualifications of possible candidates.
The selection of the nominees for the Board is made by the other members of the Board, based on our needs and the qualities required for the Board, including ethical character, integrity and maturity of judgment of the candidates; the level of experience of the candidates; their ideas regarding the material aspects of our business; the expertise of the candidates in fields relevant to us while complementing the training and experience of the other members of the Board; the will and ability of the candidates to devote the necessary time to their duties to the Board and its committees; the will of the candidates to serve on the Board for numerous consecutive financial periods; and the will of the candidates to refrain from engaging in activities which conflict with the responsibilities and duties of a director. The Board researches the training and qualifications of potential new directors which seem to correspond to the selection criteria of the Board and, depending on the results of said research, organizes meetings with the potential candidates.
In the case of incumbent directors whose terms of office are set to expire, the Board will review such directors’ overall service to us during their term of office, including the number of meetings attended, level of participation, quality of performance and any transactions of such directors with us during their term of office.
We may use various sources in order to identify the candidates for the Board, including our own contacts and the references of other directors, officers, advisors and executive placement agencies. We will consider director candidates recommended by shareholders and will evaluate those director candidates in the same manner in which we evaluate candidates recommended by other sources. In making recommendations for director nominees for the annual meeting of shareholders, we will consider any written recommendations of director candidates by shareholders received by our corporate secretary not later than 120 days before the anniversary of the previous year’s annual meeting of shareholders. Recommendations must include the candidate’s name, contact information and a statement of the candidate’s background and qualifications, and must be mailed to us. Following the selection of the candidates by the board of directors, we will propose a list of candidates to the shareholders, for our annual meeting of shareholders.
The Board does not have a separate nominating committee and has not adopted any formal written director term limit policy. Proposed nominations of director candidates are evaluated by our GHR committee.
GHR Committee
The mandate of the GHR committee consists of the evaluation of the proposed nominations of senior executives and director candidates to our Board; recommending for board approval, if appropriate; revisions of our corporate governance practices and procedures; developing new charters for any new committees established by the Board; monitoring relationships and communication between management and the Board; monitoring emerging best practices in corporate governance and oversight of governance matters; and assessing the Board and its committees. The GHR committee is also in charge of establishing the procedures which must be followed by us to comply with applicable requirements of Nasdaq regarding corporate governance.
The GHR committee has the responsibility of evaluating the compensation, performance incentives as well as the benefits granted to our management in accordance with their responsibilities and performance as well as to recommend the necessary adjustments to our Board. The GHR committee also reviews the amount and method of compensation granted to the directors. The GHR committee may retain an external firm in order to assist it during the execution of its mandate. The GHR committee considers time commitment, comparative fees, and responsibilities in determining compensation.
The GHR committee’s charter can be found on the Company’s website at https://www.acasti.com/en/investors/corporate-governance/governance-documents.
Periodic Assessments
The Board, its committees and each director are subject to periodic evaluations of their efficacy and contribution. The evaluation procedure consists of identifying any shortcomings and implementing adjustments proposed by directors at the beginning and during meetings of the Board and of each of its committees. Among other things, these adjustments deal with the level of preparation of directors, management and consultants employed by us, the relevance and sufficiency of the documentation provided to directors and the time allowed to directors for discussion and debate of items on the agenda.
Director Term Limits
The Board actively considers the issue of term limits from time to time. At this time, the Board does not believe that it is in our best interests to establish a limit on the number of times a director may stand for election. While such a limit could help create an environment where fresh ideas and viewpoints are available to the Board, a director term limit could also disadvantage us through the loss of the beneficial contribution of directors who have developed increasing knowledge of, and insight into, us and our operations over a period
82
of time. As we operate in a unique industry, it is difficult to find qualified directors with the appropriate background and experience and the introduction of a director term limit would impose further difficulty.
Policies Regarding the Representation of Women on the Board and Among Executive Officers
We have not adopted a formal written policy regarding diversity amongst executive officers and members of the Board, including mechanisms for Board renewal, in connection with, among other things, the identification and nomination of women directors. Nevertheless, we recognize that gender diversity is a significant aspect of diversity and acknowledge the important role that women with appropriate and relevant skills and experience can play in contributing to the diversity of perspective on the Board.
Rather than considering the level of representation of women for directorship and executive officer positions when making Board or executive officer appointments, we consider all candidates based on their merit and qualifications relevant to the specific role. While we recognize the benefits of diversity at all levels within our organization, we do not currently have any targets, rules or formal policies that specifically require the identification, consideration, nomination, or appointment of candidates for directorship or executive management positions or that would otherwise force the composition of our Board and executive management team.
Item 14. Principal Accounting Fees and Services Audit Fees
Our current independent registered public accounting firm is KPMG LLP, U.S. ("KPMG").
Change in Accountant
On December 11, 2023, the Audit Committee (the “Audit Committee”) of the Company's Board of Directors (the "Board") recommended to the Board and the Board approved the dismissal of Ernst & Young LLP (Canada) ("E&Y") who had been serving as the Company’s independent registered public accounting firm since February 22, 2023. The report of E&Y on the consolidated financial statements of the Company as of and for the fiscal year ended March 31, 2023 did not contain any adverse opinion or a disclaimer of opinion, nor was it qualified or modified as to uncertainty, audit scope or accounting principles. On December 11, 2023, in connection with the Company’s dismissal of E&Y, the Audit Committee recommended to the Board and the Board approved the engagement of KPMG as its new independent registered public accounting firm to audit the Company’s consolidated financial statements for the fiscal year ending March 31, 2024. The decision to engage KPMG was recommended by the Audit Committee, and approved by the Board, after taking into account KPMG’s location in the United States, the results of a competitive review process and other business factors.
Prior to our engagement of E&Y on February 22, 2023, KPMG LLP, Montreal, Quebec, Canada (“KPMG Canada”) was previously our independent registered public accounting firm. On February 22, 2023, the Audit Committee and Board approved the dismissal of KPMG Canada as our independent registered public accounting firm.
Independent Registered Public Accounting Firm Fees and Services
Audit Fees
“Audit fees” consist of fees for professional services for the audit of our annual financial statements and fees related to securities filings. Audit fees for KPMG were $300 thousand for the fiscal year ended March 31, 2024. Our previous independent registered public accounting firms were E&Y, which audited our annual financial statements for our fiscal year ended March 31, 2023, and KPMG Canada, which audited our annual financial statements for our fiscal year ended March 31, 2022. Audit fees for E&Y were CAD $143 thousand and CAD $425 thousand for the fiscal years ended March 31, 2024 and March 31, 2023, respectively. Audit fees for KPMG Canada, were nil and CAD $64 thousand for the fiscal years ended March 31, 2024 and March 31, 2023, respectively.
Audit-Related Fees
“Audit-related fees” consist of fees for professional services that are reasonably related to the performance of the audit or review of our financial statements, and which are not reported under “Audit Fees” above. KPMG Canada, billed nil and CAD $52 thousand for audit related fees for the fiscal years ended March 31, 2024, and March 31, 2023, respectively.
Tax Fees
“Tax fees” consist of fees for professional services for tax compliance, tax advice and tax planning. E&Y billed CAD $47 thousand and nil for tax fees for the fiscal years ended March 31, 2024, and March 31, 2023, respectively.
All Other Fees
83
“Other fees” include all other fees billed for professional services other than those mentioned hereinabove. We incurred no other fees for the fiscal years ended March 31, 2024 and March 31, 2023.
Pre-Approval Policies and Procedures
The Audit Committee approves all audit, audit-related services, tax services and other non-audit related services provided by the external auditors in advance of any engagement. Under the Sarbanes-Oxley Act of 2002, audit committees are permitted to approve certain fees for non-audit related services pursuant to a de minimus exception prior to the completion of an audit engagement. Non-audit related services satisfy the de minimus exception if the following conditions are met:
None of the services described above under “Principal Accounting Fees and Services” were approved by the Audit Committee pursuant to the de minimus exception.
84
PART IV
Item 15. Exhibits, Financial Statement Schedules
(a)(1) Financial Statements—The consolidated financial statements included in Item 8 are filed as part of this Annual Report on Form 10-K.
(a)(2) Financial Statement Schedules—All schedules have been omitted because they are not applicable or required, or the information required to be set forth therein is included in the consolidated financial statements or notes thereto included in Item 8 of this Annual Report on Form 10-K.
(a)(3) Exhibits—The exhibits required by Item 601 of Regulation S-K are listed in paragraph (b) below.
(b) Exhibits—The exhibits listed on the Exhibit Index below are filed herewith or are incorporated by reference to exhibits previously filed with the SEC.
EXHIBITS INDEX
Exhibit No. |
|
Description |
|
|
|
2.1 |
|
|
|
|
|
3.1 |
|
|
|
|
|
3.2 |
|
|
|
|
|
3.3 |
|
|
|
|
|
4.1 |
|
|
|
|
|
4.2 |
|
|
|
|
|
4.3 |
|
|
|
|
|
4.4 |
|
|
|
|
|
10.1+ |
|
|
|
|
|
10.2+ |
|
|
|
|
|
10.3+ |
|
|
|
|
|
10.4+ |
|
|
|
|
|
10.5+ |
|
|
|
|
|
85
10.6 |
|
|
|
|
|
10.7 |
|
|
|
|
|
16.1 |
|
|
|
|
|
16.2 |
|
|
|
|
|
21.1* |
|
|
|
|
|
23.1* |
|
Consent of KPMG LLP, an Independent Registered Public Accounting Firm. |
|
|
|
23.2* |
|
Consent of Ernst & Young LLP, an Independent Registered Public Accounting Firm. |
|
|
|
31.1* |
|
|
|
|
|
31.2* |
|
|
|
|
|
32.1* |
|
|
|
|
|
32.2* |
|
|
|
|
|
97.1* |
|
|
|
|
|
101.INS* |
|
Inline XBRL Instance Document |
|
|
|
101.SCH* |
|
Inline XBRL Taxonomy Extension Schema Document |
|
|
|
101.CAL* |
|
Inline XBRL Taxonomy Extension Calculation Linkbase Document |
|
|
|
101.DEF* |
|
Inline XBRL Taxonomy Extension Definition Linkbase Document |
|
|
|
101.LAB* |
|
Inline XBRL Taxonomy Extension Label Linkbase Document |
|
|
|
101.PRE* |
|
Inline XBRL Taxonomy Extension Presentation Linkbase Document |
|
|
|
104* |
|
Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) |
* Filed or furnished herewith.
+ Management contract, compensatory plan or arrangement.
Item 16. Form 10-K Summary
None.
86
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
Dated: June 21, 2024 |
|
|
|
ACASTI PHARMA INC. |
|
|
|
|
|
By: |
/s/ Prashant Kohli |
|
Name: |
Prashant Kohli |
|
|
Title: Chief Executive Officer and |
|
|
(Principal Executive Officer) |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Signature |
|
Title |
|
Date |
|
|
|
|
|
/s/ Prashant Kohli |
|
Chief Executive Officer |
|
June 21, 2024 |
Prashant Kohli |
|
(Principal Executive Officer) |
|
|
|
|
|
|
|
/s/ Robert DelAversano |
|
Principal Financial Officer |
|
June 21, 2024 |
Robert DelAversano |
|
(Principal Financial Officer and Principal Accounting Officer) |
|
|
|
|
|
|
|
/s/ Brian Davis |
|
Director |
|
June 21, 2024 |
Brian Davis |
|
|
|
|
|
|
|
|
|
/s/Vimal Kavuru |
|
Director |
|
June 21, 2024 |
Vimal Kavuru |
|
|
|
|
|
|
|
|
|
/s/Edward Neugeboren |
|
Director |
|
June 21, 2024 |
Edward Neugeboren |
|
|
|
|
|
|
|
|
|
/s/George Kottayil |
|
Director |
|
June 21, 2024 |
George Kottayil |
|
|
|
|
87
ACASTI PHARMA INC.
Consolidated Financial Statements
For the years ended March 31, 2024 and 2023
F-1 |
|
F-3 |
|
Consolidated Statements of Operations and Comprehensive Loss |
F-4 |
F-5 |
|
F-6 |
|
F-7 |
Report of Independent Registered Public Accounting Firm
To the Shareholders and Board of Directors
Acasti Pharma Inc.:
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheet of Acasti Pharma Inc. and subsidiaries (the Company) as of March 31, 2024, the related consolidated statements of operations and comprehensive loss, shareholders’ equity, and cash flows for the year then ended, and the related notes (collectively, the consolidated financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of March 31, 2024, and the results of its operations and its cash flows for the year then ended, in conformity with U.S. generally accepted accounting principles.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audit provides a reasonable basis for our opinion.
Critical Audit Matters
Critical audit matters are matters arising from the current period audit of the consolidated financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgments. We determined that there are no critical audit matters.
/s/ KPMG LLP
We have served as the Company’s auditor since 2023.
Philadelphia, Pennsylvania
June 21, 2024
F-1
Report of Independent Registered Public Accounting Firm
To the Shareholders and the Board of Directors of Acasti Pharma Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheet of Acasti Pharma Inc. (the “Company”) as of March 31, 2023, the related consolidated statements of operations and comprehensive loss, shareholders’ equity, and cash flows for the year ended March 31, 2023, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at March 31, 2023 and the results of its operations and its cash flows for the year ended March 31, 2023, in conformity with U.S. generally accepted accounting principles.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
/s/ Ernst & Young LLP
We served as the Company’s auditor in 2023.
Montréal, Canada
June 23, 2023, except for the effects of the reverse stock split described in Note 1, as to which the date is June 21, 2024
F-2
ACASTI PHARMA INC.
Consolidated Balance Sheets
|
|
March 31, 2024 |
|
|
March 31, 2023 |
|
||
(Expressed in thousands except share data) |
|
$ |
|
|
$ |
|
||
Assets |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
||
Current assets: |
|
|
|
|
|
|
||
Cash and cash equivalents |
|
|
|
|
|
|
||
Short-term investments |
|
|
|
|
|
|
||
Receivables |
|
|
|
|
|
|
||
Prepaid expenses |
|
|
|
|
|
|
||
Total current assets |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
||
Operating lease right of use asset |
|
|
|
|
|
|
||
Equipment, net |
|
|
|
|
|
|
||
Intangible assets |
|
|
|
|
|
|
||
Goodwill |
|
|
|
|
|
|
||
Total assets |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
||
Liabilities and Shareholders’ equity |
|
|
|
|
|
|
||
Current liabilities: |
|
|
|
|
|
|
||
Trade and other payables |
|
|
|
|
|
|
||
Operating lease liability |
|
|
|
|
|
|
||
Total current liabilities |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
||
Derivative warrant liabilities |
|
|
|
|
|
|
||
Operating lease liability |
|
|
|
|
|
|
||
Deferred tax liability |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
||
Total liabilities |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
||
Shareholders’ equity: |
|
|
|
|
|
|
||
Class A common shares, |
|
|
|
|
|
|
||
Class B, C, D and E common shares, no par value per share; unlimited shares authorized; none issued and outstanding |
|
|
|
|
|
|
||
Additional paid-in capital |
|
|
|
|
|
|
||
Accumulated other comprehensive loss |
|
|
( |
) |
|
|
( |
) |
Accumulated deficit |
|
|
( |
) |
|
|
( |
) |
Total shareholders' equity |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
||
Total liabilities and shareholders’ equity |
|
|
|
|
|
|
||
The accompanying notes are an integral part of these consolidated financial statements
F-3
ACASTI PHARMA INC.
Consolidated Statements of Operations and Comprehensive Loss
|
|
Year ended March 31, 2024 |
|
|
Year ended March 31, 2023 |
|
||
(Expressed in thousands, except share and per data) |
|
$ |
|
|
$ |
|
||
|
|
|
|
|
|
|
||
Operating expenses |
|
|
|
|
|
|
||
Research and development expenses, net of government assistance |
|
|
( |
) |
|
|
( |
) |
General and administrative expenses |
|
|
( |
) |
|
|
( |
) |
Sales and marketing |
|
|
( |
) |
|
|
( |
) |
Restructuring cost |
|
|
( |
) |
|
|
|
|
Impairment of intangible assets |
|
|
|
|
|
( |
) |
|
Impairment of goodwill |
|
|
|
|
|
( |
) |
|
Impairment of assets held for sale |
|
|
|
|
|
( |
) |
|
Loss from operating activities |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
|
||
Foreign exchange loss |
|
|
( |
) |
|
|
( |
) |
Change in fair value of derivative warrant liabilities |
|
|
( |
) |
|
|
|
|
Interest income and other expense, net |
|
|
|
|
|
|
||
Total other income (expense), net |
|
|
( |
) |
|
|
|
|
Loss before income tax benefit |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
|
||
Income tax benefit |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
||
Net loss and total comprehensive loss |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
|
||
Basic and diluted loss per share |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
|
||
Weighted average number of shares outstanding |
|
|
|
|
|
|
||
The accompanying notes are an integral part of these consolidated financial statements
F-4
ACASTI PHARMA INC.
|
|
Class A common shares |
|
|
Additional paid-in capital |
|
|
Accumulated other comprehensive loss |
|
|
Accumulated deficit |
|
|
Total shareholders' equity |
|
|||||||||
(Expressed in thousands expect share data) |
|
Number |
|
|
Amount |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||||
Balance, March 31, 2023 |
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
|
||||
Net loss and total comprehensive loss for the period |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Issuance of common shares and pre-funded warrants through private placement, net of offering costs |
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||||
Issuance of common shares upon the exercise of stock options |
|
|
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|||
Balance at March 31, 2024 |
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
|
||||
|
|
Class A common shares |
|
|
Additional paid-in capital |
|
|
Accumulated other |
|
|
Accumulated deficit |
|
|
Total shareholders' equity |
|
|||||||||
(Expressed in thousands expect share data) |
|
Number |
|
|
Amount |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||||
Balance, March 31, 2022 |
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
|
||||
Net loss and total comprehensive loss for the period |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
Cumulative translation adjustment |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
— |
|
|
|
( |
) |
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Net proceeds from shares issued under the at-the-market (ATM) program |
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|||
Balance at March 31, 2023 |
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
|
||||
The accompanying notes are an integral part of these consolidated financial statements
F-5
ACASTI PHARMA INC.
Consolidated Statements of Cash Flows
|
|
Year ended March 31, 2024 |
|
|
Year ended March 31, 2023 |
|
||
|
|
$ |
|
|
$ |
|
||
Cash flows used in operating activities: |
|
|
|
|
|
|
||
Net loss |
|
|
( |
) |
|
|
( |
) |
Adjustments: |
|
|
|
|
|
|
||
Depreciation of equipment |
|
|
|
|
|
|
||
Gain on sale of equipment |
|
|
( |
) |
|
|
|
|
Impairment of intangible assets |
|
|
|
|
|
|
||
Impairment of goodwill |
|
|
|
|
|
|
||
Impairment of assets held for sale |
|
|
|
|
|
|
||
Stock-based compensation |
|
|
|
|
|
|
||
Change in fair value of warrant liabilities |
|
|
|
|
|
( |
) |
|
Deferred income tax benefit |
|
|
( |
) |
|
|
( |
) |
Unrealized foreign exchange (gain) loss |
|
|
|
|
|
|
||
Loss on disposal |
|
|
|
|
|
|
||
Changes in operating assets and liabilities: |
|
|
|
|
|
|
||
Receivables |
|
|
|
|
|
( |
) |
|
Prepaid expenses |
|
|
|
|
|
|
||
Trade and other payables |
|
|
( |
) |
|
|
|
|
Operating lease right of use asset |
|
|
( |
) |
|
|
|
|
Net cash used in operating activities |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
|
||
Cash flows from investing activities: |
|
|
|
|
|
|
||
Purchase of equipment |
|
|
( |
) |
|
|
( |
) |
Proceeds from sale of equipment |
|
|
|
|
|
|
||
Purchase of short-term investments |
|
|
( |
) |
|
|
( |
) |
Maturity of short-term investments |
|
|
|
|
|
|
||
Net cash provided by investing activities |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
||
Cash flows from financing activities: |
|
|
|
|
|
|
||
Net proceeds from issuance of common shares and warrants from private placement |
|
|
|
|
|
|
||
Proceeds from issuance of common shares from exercise of stock options |
|
|
|
|
|
|
||
Net proceeds from shares issued under the at-the-market (ATM) program |
|
|
|
|
|
|
||
Net cash provided by financing activities |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
||
Effect of exchange rate fluctuations on cash and cash equivalents |
|
|
|
|
|
( |
) |
|
|
|
|
|
|
|
|
||
Net decrease in cash and cash equivalents |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
|
||
Cash and cash equivalents, beginning of year |
|
|
|
|
|
|
||
Cash and cash equivalents, end of year |
|
|
|
|
|
|
||
Cash and cash equivalents are comprised of: |
|
|
|
|
|
|
||
Cash |
|
|
|
|
|
|
||
Cash equivalents |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
||
F-6
ACASTI PHARMA INC.
Notes to the Consolidated Financial Statements
(Expressed in thousands except share and per share data)
1. Nature of Operations
Acasti Pharma Inc. (“Acasti” or the “Company”) is incorporated under the Business Companies Act (Québec) (formerly Part 1A of the Companies Act (Québec)). The Company is domiciled in Canada and its principal executive office is located at 103 Carnegie Center Suite 300 Princeton, New Jersey 08540.
The Company’s Class A common shares, no par value per share (“Common Shares”), are listed on the Nasdaq Capital Market (“Nasdaq”) and, through March 27, 2023, the Company's Common Shares were also listed on the TSX Venture Exchange (“TSXV”), in each case, under the symbol “ACST”. On March 13, 2023, the Company received approval to voluntarily delist from the TSXV. Effective as at the close of trading on March 27, 2023, the Company's Common Shares are no longer listed and posted for trading on the TSXV.
In August 2021, the Company completed the acquisition via a share-for-share merger of Grace Therapeutics, Inc. (“Grace Therapeutics”), a privately held emerging biopharmaceutical company focused on developing innovative drug delivery technologies for the treatment of rare and orphan diseases. The post-merger Company is focused on building a late-stage specialty pharmaceutical company specializing in rare and orphan diseases and developing and commercializing products that improve clinical outcomes using its novel drug delivery technologies. The Company seeks to apply new proprietary formulations to existing pharmaceutical compounds to achieve enhanced efficacy, faster onset of action, reduced side effects, more convenient delivery and increased patient compliance; all of which could result in improved patient outcomes. The active pharmaceutical ingredients chosen by the Company for further development may be already approved in the target indication or could be repurposed for use in new indications.
The Company has incurred operating losses and negative cash flows from operations in each year since its inception. The Company expects to incur significant expenses and continued operating losses for the foreseeable future.
In May 2023, the Company implemented a strategic realignment plan to enhance shareholder value that resulted in the Company engaging a new management team, streamlining its research and development activities and greatly reducing its workforce. Following the realignment, the Company is a smaller, more focused organization, based in the United States, and concentrated on its development of its lead product GTX-104. Further development of GTX-102 and GTX-101 will occur at such time when the Company is able to secure additional funding, or enters into strategic partnerships for license or sale with third parties.
On September 24, 2023, the Company entered into a securities purchase agreement with certain institutional and accredited investors. Gross proceeds to the Company from this private placement were approximately $
The Company will require additional capital to fund its daily operating needs beyond that time. The Company does not expect to generate revenue from product sales unless and until it successfully completes drug development and obtains regulatory approval, which the Company expects will take several years and is subject to significant uncertainty. To date, the Company has financed its operations primarily through public offerings and private placements of its Common Shares, warrants and convertible debt and the proceeds from research tax credits. Until such time that the Company can generate significant revenue from drug product sales, if ever, it will require additional financing, which is expected to be sourced from a combination of public or private equity or debt financing or other non-dilutive sources, which may include fees, milestone payments and royalties from collaborations with third parties. Arrangements with collaborators or others may require the Company to relinquish certain rights related to its technologies or drug product candidates. Adequate additional financing may not be available to the Company on acceptable terms, or at all. The Company’s inability to raise capital as and when needed could have a negative impact on its financial condition and its ability to pursue its business strategy. The Company plans to raise additional capital in order to maintain adequate liquidity. Negative results from studies or trials, if any, or depressed prices of the Company’s stock could impact the Company’s ability to raise additional financing. Raising additional equity capital is subject to market conditions that are not within the Company’s control. If the Company is unable to raise additional funds, the Company may not be able to realize its assets and discharge its liabilities in the normal course of business.
The Company remains subject to risks similar to other development stage companies in the biopharmaceutical industry, including
F-7
compliance with government regulations, protection of proprietary technology, dependence on third-party contractors and consultants and potential product liability, among others.
Reverse stock split
On June 29, 2023, the Board of Directors of the Company approved an amendment to the Company's Articles of Incorporation to implement a reverse stock split of the Company's Common Shares, at a ratio of
2. Summary of significant accounting policies
Basis of presentation
These consolidated financial statements of Acasti Pharma Inc., which include the accounts of its subsidiaries, have been prepared in accordance with generally accepted accounting principles in the United States of America ("U.S. GAAP"). All intercompany transactions and balances are eliminated on consolidation.
Use of estimates
The preparation of the consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, income, and expenses. Actual results may differ from these estimates.
Estimates are based on management’s best knowledge of current events and actions that management may undertake in the future. Estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimates are revised and in any future periods affected.
Estimates and assumptions include the measurement of stock-based compensation, derivative warrant liabilities, accruals for research and development contracts and contract organization agreements, and valuation of intangibles and goodwill. Estimates and assumptions are also involved in determining the extent to which research and development expenses qualify for research and development tax credits. The Company recognizes tax credits once it has reasonable assurance that they will be realized.
Cash equivalents
Cash equivalents comprise of highly liquid investments purchased with original maturities of 90 days or less. Cash equivalents consist of guaranteed investment certificates.
Equipment
Equipment is measured at cost less accumulated depreciation and accumulated impairment losses, if any. Cost includes expenditures that are directly attributable to the acquisition of the asset, including all costs incurred in bringing the asset to its present location and condition. Gains and losses on disposal of equipment are determined by comparing the proceeds from disposal with the carrying amount of equipment.
Depreciation is recognized on a declining basis over the estimated useful lives of equipment, since this most closely reflects the expected pattern of consumption of the future economic benefits embodied in the asset. Items of equipment are depreciated from the date that they are available for use or, in respect of assets not yet in service, from the date they are ready for their intended use.
Intangible assets - acquired in-process research and development
In a business combination, the fair value of in-process research and development (“IPR&D”) acquired is capitalized and accounted for as indefinite-lived intangible assets, and not amortized until the underlying project receives regulatory approval, at which point the intangible assets will be accounted for as definite-lived intangible assets and amortized over the remaining useful life or discontinued. If discontinued, the intangible asset will be written off. Research and development (“R&D”) costs incurred after the acquisition are expensed as incurred.
The estimated fair values of identifiable intangible assets were determined using the multi-period excess earnings method, which is a valuation methodology that provides an estimate of the fair value of an asset based on market participant expectations of the cash flows an asset would generate over its remaining useful life. The significant assumptions used in the valuation are the discount rate, the probability of clinical success of research and development programs, obtaining regulatory approval and forecasted net sales, including milestone payments and royalty revenues.
F-8
Impairment of long-lived assets
The Company reviews the recoverability of its finite long-lived assets whenever events or changes in circumstances indicate that it is carrying amount may not be recoverable. The carrying amount is first compared with the undiscounted cash flows. If the carrying amount is higher than the sum of undiscounted cash flows, then the Company determines the fair value of the underlying asset group. Any impairment loss to be recognized is measured as the difference by which the carrying amount of the asset group exceeds the estimated fair value of the asset group.
Goodwill and indefinite-lived assets are not amortized but are subject to an impairment review annually and more frequently when indicators of impairment exist. An impairment of goodwill could occur if the carrying amount of a reporting unit exceeds the fair value of that reporting unit. An impairment of indefinite-lived intangible assets would occur if the fair value of the intangible asset is less than the carrying value.
The Company tests its goodwill for impairment by first assessing qualitative factors to determine whether it is more likely than not that the fair value is less than its carrying amount. If the Company concludes it is more likely than not that fair value of the reporting unit is less than its carrying amount, a quantitative impairment test is performed.
The Company tests indefinite-lived intangible assets for impairment by first assessing qualitative factors to determine whether it is more likely than not that the fair value is less than its carrying amount. If the Company concludes it is more likely than not that the fair value is less than it's carrying amount, a quantitative impairment test is performed. The Company's annual impairment test is performed in the fourth quarter of the fiscal year.
Research and development costs
Research and developments expenditures are expensed as incurred. These costs consist of employees’ salaries and benefits related to research and development activities, contractors and consultants that conduct the Company’s clinical trials, laboratory material and small equipment, clinical trial materials, stock-based compensation expense, and other non-clinical costs and regulatory fees. Advance payments for goods and services that will be used in future research and development are recognized in prepaids or other assets and are expensed when the services are performed, or the goods are used.
Stock-based compensation
The Company has in place a stock option plan for directors, officers, employees, and consultants of the Company, with grants under the stock option plan approved by the Company’s Board of Directors. The plan provides for the granting of options to purchase Common Shares and the exercise price of each option equals the closing trading price of Common Shares on the day prior to the grant. The Company accounts for stock-based compensation arrangements in accordance with provisions of Accounting Standards Codification (“ASC”) 718, Compensation—Stock Compensation. ASC 718 requires the recognition of compensation expense, using a fair-value based method, for costs related to all share-based payments including stock options. ASC 718 requires companies to estimate the fair value of share-based payment awards on the date of grant using an option-pricing model. The Company measures the cost of such awards based on the fair value of the award at grant date and recognizes stock-based compensation expense in the Consolidated Statements of Operations and Comprehensive Loss on a tranche by tranche basis. The fair value of options is estimated for each tranche of an award that vests on a graded basis. The fair value of options is estimated using the Black-Scholes option pricing model, which uses various inputs including fair value of the Common Shares at the grant date, expected term, historical volatility, risk-free interest rate and expected dividend yields of the Common Shares. The Company applies an estimated forfeiture rate derived from historical employee termination behavior in determining compensation expense. If the actual forfeitures differ from those estimated by management, adjustment to compensation expense may be required in future periods.
Government grants
Government grants are recorded as a reduction of the related expense or cost of the asset acquired. Government grants are recognized when there is reasonable assurance that the Company has met the requirements of the approved grant program and there is reasonable assurance that the grant will be received.
Leases
At the inception of an arrangement, the Company determines whether the arrangement is or contains a lease based on the unique facts and circumstances present in the arrangement. Operating lease liabilities and their corresponding right-of-use assets are initially recorded based on the present value of lease payments over the expected remaining lease term. Certain adjustments to the right-of-use asset may be required for items such as incentives received. The interest rate implicit in lease contracts is typically not readily determinable. As a result, the Company utilizes its incremental borrowing rate to discount lease payments, which reflects the fixed rate at which the Company could borrow on a collateralized basis the amount of the lease payments in the same currency, for a similar term, in a similar
F-9
economic environment. The Company has elected not to recognize leases with an original term of one year or less on the balance sheet. The Company typically only includes an initial lease term in its assessment of a lease arrangement. Options to renew a lease are not included in the Company’s assessment unless there is reasonable certainty that the Company will renew. The Company’s lease expense is recognized in research and development expenses. The Company does not have financing leases.
In accordance with FASB ASC 842—Leases (“Topic 842”), components of a lease should be split into three categories: lease components, non-lease components and non-components. The fixed and in-substance fixed contract consideration (including any consideration related to non-components) must be allocated based on the respective relative fair values to the lease components and non-lease components. Entities may elect not to separate lease and non-lease components. The Company has elected to account for lease and non-lease components together as a single lease component for all underlying assets and allocate all of the contract consideration to the lease component only.
Income taxes
Income taxes comprises current and deferred taxes. The provision for income taxes is computed using the asset and liability method.
Current tax is the expected tax payable or receivable on the taxable income or loss for the year, using tax rates enacted at the reporting date, and any adjustment to tax payable in respect of previous years.
Deferred tax is recognized in respect of temporary differences between the carrying amounts (tax base) of assets and liabilities for financial reporting purposes and the amounts used for taxation purposes. Deferred tax assets and liabilities are measured at the tax rate expected to apply when the underlying asset or liability is realized (settled) based on the rates that are enacted at the reporting date. Deferred tax assets and liabilities are offset if the Company has the right to set off the amount owed by with the amount owed by the other party, the Company intends to set off and the offset right is enforceable at law. A deferred tax asset is recognized for unused tax losses, and tax credits, reduced by a valuation allowance. A valuation allowance is recorded to reduce the carrying amount of deferred income tax assets when it is more likely than not that these assets will not be realized. tax benefits related to tax positions not deemed to meet the “more-likely-than-not” threshold are not permitted to be recognized in the consolidated financial statements.
Earnings per share
The Company presents basic and diluted earnings per share ("EPS") data for its Common Shares. Basic EPS is calculated by dividing the net income or loss attributable to the holders of Common Shares by the weighted average number of Common Shares outstanding during the year. Diluted EPS is determined by adjusting the net income or loss attributable to the holders of Common Shares and the weighted average number of Common Shares outstanding adjusted for the effects of all dilutive potential Common Shares, which comprise warrants and share options granted to employees. The basic and diluted EPS are the same due to loss position.
Segment reporting
An operating segment is a component of the Company that engages in business activities from which it may earn revenues and incur expenses. The Company has
Derivative warrant liabilities
Derivative warrant liabilities are recognized initially at fair value. Subsequent to initial recognition, derivative warrant liabilities are measured at fair value, with changes in fair value are recognized in the Consolidated Statement of Operations and Comprehensive Loss.
Fair value measurements
Certain of the Company’s accounting policies and disclosures require the determination of fair value, for both financial assets and liabilities.
In establishing fair value, the Company uses a fair value hierarchy based on levels as defined below:
The Company has determined that the carrying values of its short-term financial assets and liabilities (cash and cash equivalents, short-term investments and trade and other payables) approximate their fair value given the short-term nature of these instruments. The Company measured its derivative warrant liabilities at fair value on a recurring basis using level 3 inputs.
F-10
Financial Instruments
Concentration of credit risk
Financial instruments that potentially subject the Company to a concentration of credit risk consist primarily of cash and cash equivalents. Cash and cash equivalents are all invested in accordance with the Company’s Investment Policy with the primary objective being the preservation of capital and the maintenance of liquidity, which risk is managed by dealing only with highly rated Canadian and U.S. institutions. The Company maintains its cash and cash equivalents at accredited financial institutions in amounts that exceed federally insured limits. The Company does not believe that it is subject to unusual credit risk beyond the normal credit risk associated with commercial banking relationships.
Recent accounting pronouncements
The Company has considered recent accounting pronouncements and concluded that they are either not applicable to the business or that the effect is not expected to be material to the consolidated financial statements as a result of future adoption.
3. Fair value measurements
Assets and liabilities measured at fair value on a recurring basis as of March 31, 2024 are as follows:
|
|
Total |
|
|
Quoted prices in active markets (Level 1) |
|
|
Significant other observable inputs (Level 2) |
|
|
Significant unobservable inputs (Level 3) |
|
|
||||
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|
||||
Assets |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Guaranteed investment certificates and term deposits |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Total assets |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Derivative warrant liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Total liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Assets measured at fair value on a recurring basis as of March 31, 2023 are as follows:
|
|
Total |
|
|
Quoted prices in active markets (Level 1) |
|
|
Significant other observable inputs (Level 2) |
|
|
Significant unobservable inputs (Level 3) |
|
|
||||
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|
||||
Assets |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Term deposits classified as cash equivalents |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Guaranteed investment certificate classified as a |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Total assets |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
There were changes in valuation techniques or transfers between Levels 1, 2 or 3 during the years ended March 31, 2024 and 2023. The Company’s derivative warrant liabilities are measured at fair value on a recurring basis using unobservable inputs that are classified as Level 3 inputs. Refer to Note 10(b) for the valuation techniques and assumptions used in estimating the fair value of the derivative warrant liabilities.
F-11
4. Receivables
|
|
March 31, 2024 |
|
|
March 31, 2023 |
|
||
|
|
$ |
|
|
$ |
|
||
Sales tax receivables |
|
|
|
|
|
|
||
Government assistance |
|
|
|
|
|
|
||
Interest receivable |
|
|
|
|
|
|
||
Other receivable |
|
|
|
|
|
|
||
Total receivables |
|
|
|
|
|
|
||
5. Equipment
The following is a summary of equipment, net:
March 31, 2024 |
|
Cost |
|
|
Accumulated |
|
|
Write-off |
|
|
Net book |
|
||||
|
|
$ |
|
|
$ |
|
|
|
|
|
$ |
|
||||
Furniture and office equipment |
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
|
||
Computer equipment |
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
|
||
Laboratory equipment |
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
|
||
Software |
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
|
||
March 31, 2023 |
|
Cost |
|
|
Accumulated |
|
|
Write-off |
|
|
Net book |
|
||||
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
Furniture and office equipment |
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
|
||
Computer equipment |
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
|
||
Laboratory equipment |
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
|
||
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
|
||
Depreciation expense was $
6. Intangible assets and goodwill
Individual IPR&D projects and goodwill are tested for impairment on an annual basis in the fourth quarter, and in between annual tests if an event occurs or circumstances change that would more likely than not reduce the fair value of each technology or our reporting unit below its carrying value. In April 2023, the Company announced its strategic realignment plan to prioritize resources to GTX-104, from GTX-101 and GTX-102 triggering a comprehensive review as of March 31, 2023. The estimated fair values of identifiable intangible assets were determined using the multi-period excess earnings method.
|
|
GTX-104 |
|
GTX-102 |
|
GTX-101 |
|
Total |
|
||||
|
|
$ |
|
$ |
|
$ |
|
$ |
|
||||
Intangible assets – in-process research and development |
|
|
|
|
|
|
|
|
|
||||
Balance, March 31, 2022 |
|
|
|
|
|
|
|
|
|
||||
Impairment |
|
|
|
|
( |
) |
|
( |
) |
|
( |
) |
|
Balance, March 31, 2023 |
|
|
|
|
|
|
|
|
|
||||
Impairment |
|
|
|
|
|
|
|
|
|
||||
Balance, March 31, 2024 |
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
||||
During 2023,
F-12
|
|
$ |
|
|
Goodwill |
|
|
|
|
Balance, March 31, 2022 |
|
|
|
|
Impairment |
|
|
( |
) |
Balance, March 31, 2023 |
|
|
|
|
Impairment |
|
|
|
|
Balance, March 31, 2024 |
|
|
|
|
The multi-period excess earnings method models used to estimate the fair value of assets of our IPR&D reflect significant assumptions and are level 3 unobservable data regarding the estimates a market participant would make in order to evaluate a drug development asset, including the following:
The Company's IPR&D projects, consistent with others in our industry, have risks and uncertainties associated with the timely and successful completion of the development and commercialization of product candidates, including our ability to confirm safety and efficacy based on data from clinical trials, our ability to obtain necessary regulatory approvals and our ability to successfully complete these tasks within budgeted costs. It is not permitted to market a human therapeutic without obtaining regulatory approvals, and such approvals require the completion of clinical trials that demonstrate that a product candidate is safe and effective. In addition, the availability and extent of coverage and reimbursement from third-party payers, including government healthcare programs and private insurance plans as well as competitive product launches, affect the revenues a product can generate. Consequently, the eventual realized values, if any, of acquired IPR&D projects may vary from their estimated fair values.
7. Trade and other payables
|
|
March 31, 2024 |
|
|
March 31, 2023 |
|
||
|
|
$ |
|
|
$ |
|
||
Trade payables |
|
|
|
|
|
|
||
Accrued liabilities and other payables |
|
|
|
|
|
|
||
Employee salaries and benefits payable |
|
|
|
|
|
|
||
Total trade and other payables |
|
|
|
|
|
|
||
8. Leases
The Company has historically entered into lease arrangements for its research and development and quality control laboratory facility located in Sherbrooke, Québec.
Supplemental balance sheet information related to leases was as follows:
|
|
March 31, 2024 |
|
|
March 31, 2023 |
|
||
|
|
$ |
|
|
$ |
|
||
Operating lease right of use asset |
|
|
|
|
|
|
||
Operating lease liability, current |
|
|
|
|
|
|
||
Operating lease liability, long-term |
|
|
|
|
|
|
||
Total operating lease liability |
|
|
|
|
|
|
||
Supplemental lease expense related to leases is as follows:
F-13
|
|
Year ended March 31, 2024 |
|
|
Year ended March 31, 2023 |
|
||
|
|
$ |
|
|
$ |
|
||
Operating lease cost |
|
|
|
|
|
|
||
Total lease expense |
|
|
|
|
|
|
||
The following table contains a summary of the lease costs recognized under ASC 842 and other information pertaining to the Company’s operating lease for the year ended March 31, 2024:
Operating cash flows for operating lease |
|
$ |
|
|
Weighted-average remaining lease term (in years) |
|
|
|
|
Weighted-average discount rate |
|
|
% |
As the Company's lease does not provide an implicit rate, the Company utilized its incremental borrowing rate to discount lease payments, which reflects the fixed rate at which the Company could borrow on a collateralized basis the amount of the lease payments in the same currency, for a similar term, in a similar economic environment. As of March 31, 2024, there were no future minimum lease payments.
9. Shareholders' equity
Common Shares
Authorized capital stock
Private Placement
In September 2023, the Company entered into a securities purchase agreement (the “Purchase Agreement”) with certain institutional and accredited investors in connection with a private placement of the Company's securities (the “Offering”). Pursuant to the Purchase Agreement, the Company agreed to offer and sell
F-14
The Offering closed on September 25, 2023. The Offering included the issuance of Common Shares, Pre-funded Warrants, and Common Warrants to related parties Shore Pharma LLC, an entity that was controlled by Vimal Kavuru, the Chair of our Board of Directors, at the time of the Offering and SS Pharma LLC, resulting in proceeds of $
At-the-Market (“ATM”) Program
In June 2020, the Company entered into an amended and restated sales agreement (the “Sales Agreement”) with B. Riley FBR, Inc. (“B.Riley”), Oppenheimer & Co. Inc. and H.C. Wainwright & Co., LLC (collectively, the “Agents”) to amend the Company’s existing ATM program. Under the terms of the Sales Agreement, which had a three-year term, the Company could issue and sell from time to time, Common Shares having aggregate gross proceeds of up to $
During the year ended March 31, 2024,
Warrants
On May 9, 2023, warrants issued pursuant to the Company’s May 2018 Canadian public offering to acquire
As further discussed above, on September 25, 2023, the Company issued Warrants exercisable for
The Common Warrants issued as a part of the Offering are derivative warrant liabilities given the warrant indenture did not meet the fixed-for-fixed criterion and that the Common Warrants are not indexed to the Company’s own stock. Proceeds were allocated amongst Common Shares, Pre-funded Warrants, and Common Warrants by applying the residual method, with fair value of the Common Warrants determined using the Black-Scholes model, resulting in an initial warrant liability of $
The derivative warrant liabilities are measured at fair value at each reporting period and the reconciliation of changes in fair value is presented in the following table:
|
|
March 31, 2024 |
|
March 31, 2023 |
|
||
|
|
$ |
|
$ |
|
||
Beginning balance |
|
|
|
|
|
||
Issued during the year |
|
|
|
|
|
||
|
|
|
|
( |
) |
||
Ending balance |
|
|
|
|
|
||
The warrant liability was determined based on the fair value of warrants at the issue date and the reporting dates using the Black-Scholes model with the following weighted-average assumptions will expire on the earlier of (i) the 60th day after the date of the acceptance by the U.S. Food and Drug Administration of a New Drug Application for the Company's product candidate GTX-104 or (ii) five years from the date on issuance.
F-15
|
|
|
|
|
|
|
||
|
|
September 25, 2023 |
|
|
March 31, 2024 |
|
||
Risk-free interest rate |
|
|
% |
|
|
% |
||
Share price |
|
$ |
|
|
$ |
|
||
Expected warrant life |
|
|
|
|
|
|
||
Dividend yield |
|
|
% |
|
|
% |
||
Expected volatility |
|
|
% |
|
|
% |
||
The weighted-average assumptions were prorated based on the probability of the warrant liability expiring on the 60th day after the date of the acceptance by the U.S. Food and Drug Administration of a New Drug Application for the Company's product candidate GTX-104 and of it expiring on five years from the date of issuance. The weighted-average fair values of the Common Warrants were determined to be $
At March 31, 2024, the Company had outstanding Common Warrants to purchase
During the years ended March 31, 2024 and 2023,
10. Stock-based compensation
At March 31, 2024, the Company had in place a stock option plan for directors, officers, employees, and consultants of the Company (“Stock Option Plan”). As of March 31, 2024, there were
The Stock Option Plan provides for the granting of options to purchase Common Shares. Under the terms of the Stock Option Plan, the exercise price of the stock options granted under the Stock Option Plan may not be lower than the closing price of the Company’s Common Shares on the Nasdaq Capital Market at the close of such market the day preceding the grant. The maximum number of Common Shares that may be issued upon exercise of options granted under the amended Stock Option Plan shall not exceed 20% of the aggregate number of issued and outstanding shares of the Company as of July 28, 2022. The terms and conditions for acquiring and exercising options are set by the Company’s Board of Directors, subject to, among others, the following limitations: the term of the options cannot exceed
The total number of options issued to any one consultant within any twelve-month period cannot exceed
The following table summarizes information about activities within the Stock Option Plan for the year ended March 31, 2024:
|
|
Number of |
|
|
Weighted average |
|
|
Remaining Contractual Term (years) |
|
|
Aggregate Intrinsic Value (in thousands) |
|
||||
|
|
|
|
|
$ |
|
|
|
|
|
$ |
|
||||
Outstanding, March 31, 2023 |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Granted |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Exercised |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|||
Forfeited/Cancelled |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|||
Outstanding, March 31, 2024 |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Exercisable, March 31, 2024 |
|
|
|
|
|
|
|
|
|
|
|
|
||||
F-16
Forfeited and cancelled options were as a result of the Company's restructuring that occurred during the year ended March 31, 2024. On July 14, 2023, the Company's Board of Directors approved the grant of options to purchase
The weighted-average grant date fair value of awards for options granted during the years ended March 31, 2024 and 2023 was $
|
|
March 31, 2024 |
|
|
March 31, 2023 |
|
||
|
|
Weighted-average |
|
|
Weighted-average |
|
||
Exercise price |
|
$ |
|
|
$ |
|
||
Share price |
|
$ |
|
|
$ |
|
||
Dividend |
|
|
|
|
|
|
||
Risk-free interest |
|
|
% |
|
|
% |
||
Estimated life (years) |
|
|
|
|
|
|
||
Expected volatility |
|
|
% |
|
|
% |
||
Stock-based compensation expense recognized under ASC 718 related to the stock option plan is summarized as follows:
|
|
March 31, 2024 |
|
|
March 31, 2023 |
|
||
|
|
$ |
|
|
$ |
|
||
Research and development expenses |
|
|
|
|
|
|
||
General and administrative expenses |
|
|
|
|
|
|
||
Sales and marketing expenses |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
||
As of March 31, 2024, there was $
Equity incentive plan
The Company established an equity incentive plan (the “Equity Incentive Plan”) for employees, directors, and consultants. The Equity Incentive Plan provides for the issuance of
11. Loss per share
The Company has generated a net loss for all periods presented, therefore diluted loss per share is the same as basic loss per share since the inclusion of potentially dilutive securities would have had an anti-dilutive effect. All currently outstanding options and warrants could potentially be dilutive in the future.
The Company excluded the following potential Common Shares, presented based on amounts outstanding at each period end, from the computation of diluted net loss per share attributable to common shareholders for the periods indicated because including them would have had an anti-dilutive effect:
|
|
March 31, 2024 |
|
|
March 31, 2023 |
|
||
Options outstanding |
|
|
|
|
|
|
||
September 2023 Common Warrants |
|
|
|
|
|
|
||
May 2018 public offering warrants |
|
|
|
|
|
|
||
Basic and diluted net loss per share is calculated based upon the weighted-average number of Common Shares outstanding during the period. Common Shares underlying the Pre-funded Warrants are included in the calculation of basic and diluted earnings per share.
F-17
12. Income taxes
Income tax (benefit) expense:
|
|
Year ended March 31, 2024 |
|
|
Year ended March 31, 2023 |
|
||
|
|
$ |
|
|
$ |
|
||
Current tax (benefit) expense |
|
|
|
|
|
|
||
Deferred tax (benefit) expense |
|
|
( |
) |
|
|
( |
) |
Income tax (benefit) expense |
|
|
( |
) |
|
|
( |
) |
A reconciliation between tax expense and the product of accounting income multiplied by the basic income tax rate for the years ended March 31, 2024 and 2023 is as follows:
|
|
Year ended March 31, 2024 |
|
|
Year ended March 31, 2023 |
|
||
|
|
$ |
|
|
$ |
|
||
Tax at Canadian Rate |
|
|
( |
) |
|
|
( |
) |
Difference in foreign tax rates |
|
|
( |
) |
|
|
( |
) |
Non-deductible stock-based compensation |
|
|
|
|
|
|
||
Non-deductible change in fair value of warrants |
|
|
|
|
|
( |
) |
|
Non-deductible transaction costs |
|
|
|
|
|
|
||
Non-deductible goodwill impairment |
|
|
|
|
|
|
||
Other non-deductible items |
|
|
|
|
|
|
||
Non-refundable federal investment tax credit |
|
|
|
|
|
( |
) |
|
State non income taxes |
|
|
|
|
|
|
||
Change in tax rates |
|
|
( |
) |
|
|
|
|
Change in valuation allowance |
|
|
|
|
|
|
||
Other |
|
|
|
|
|
( |
) |
|
Total tax (benefit) expense |
|
|
( |
) |
|
|
( |
) |
Net deferred income tax assets as of March 31, 2024, and 2023 were comprised of the following:
|
|
March 31, 2024 |
|
|
March 31, 2023 |
|
||
|
|
$ |
|
|
$ |
|
||
Deferred income tax assets |
|
|
|
|
|
|
||
Tax losses carried forward |
|
|
|
|
|
|
||
Research and development expenses |
|
|
|
|
|
|
||
Property, plant and equipment |
|
|
|
|
|
|
||
Intangible assets |
|
|
|
|
|
|
||
Operating lease right of use liability |
|
|
|
|
|
|
||
Financing expenses |
|
|
|
|
|
|
||
Net federal investment tax credits |
|
|
|
|
|
|
||
Other deductible temporary differences |
|
|
|
|
|
|
||
Total deferred income tax assets |
|
|
|
|
|
|
||
Valuation allowance |
|
|
( |
) |
|
|
( |
) |
Deferred income tax liabilities |
|
|
|
|
|
|
||
Intangible assets |
|
|
( |
) |
|
|
( |
) |
Operating lease asset |
|
|
|
|
|
( |
) |
|
Other taxable temporary differences |
|
|
|
|
|
( |
) |
|
Deferred tax liabilities |
|
|
( |
) |
|
|
( |
) |
Net deferred tax liabilities |
|
|
( |
) |
|
|
( |
) |
As at March 31, 2024, the amounts and expiry dates of tax attributes and temporary differences, which are available to reduce future
F-18
years’ taxable income, were as follows:
|
|
|
|
|
|
|
|
March 31, 2024 |
|
|||
|
|
Federal |
|
|
Provincial |
|
|
United States |
|
|||
|
|
$ |
|
|
$ |
|
|
$ |
|
|||
Tax losses carried forward |
|
|
|
|
|
|
|
|
|
|||
2028 |
|
|
|
|
|
|
|
|
|
|||
2029 |
|
|
|
|
|
|
|
|
|
|||
2030 |
|
|
|
|
|
|
|
|
|
|||
2031 |
|
|
|
|
|
|
|
|
|
|||
2032 |
|
|
|
|
|
|
|
|
|
|||
2033 |
|
|
|
|
|
|
|
|
|
|||
2034 |
|
|
|
|
|
|
|
|
|
|||
2035 |
|
|
|
|
|
|
|
|
|
|||
2036 |
|
|
|
|
|
|
|
|
|
|||
2037 |
|
|
|
|
|
|
|
|
|
|||
2038 |
|
|
|
|
|
|
|
|
|
|||
2039 |
|
|
|
|
|
|
|
|
|
|||
2040 |
|
|
|
|
|
|
|
|
|
|||
2041 |
|
|
|
|
|
|
|
|
|
|||
2042 |
|
|
|
|
|
|
|
|
|
|||
2043 |
|
|
|
|
|
|
|
|
|
|||
2044 |
|
|
|
|
|
|
|
|
|
|||
No expiry |
|
|
|
|
|
|
|
|
|
|||
Total |
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|||
Research and development expenses, without time limitation |
|
|
|
|
|
|
|
|
|
|||
Scientific Research & Experimental Development Expenditures investment tax credit carryforwards |
|
|
|
|
|
|
|
|
|
|||
Unrecognized tax benefits
The Company does not expect a significant change to the amount of unrecognized tax benefits over the next 12 months. However, any adjustments arising from certain ongoing examinations by tax authorities could alter the timing or amount of taxable income or deductions, of the allocation of income among tax jurisdictions, and these adjustments could differ from the amount accrued. The Company’s federal and provincial income tax returns filed for all years remain subject to examination by the taxation authorities.
Government assistance
Government assistance is comprised of research and development investment tax credits receivable from the Quebec provincial government which relate to qualifiable research and development expenditures under the applicable tax laws. The amounts recorded as receivables are subject to a government tax audit and the final amounts received may differ from those recorded.
|
|
March 31, 2024 |
|
|
March 31, 2023 |
|
||
|
|
$ |
|
|
$ |
|
||
Investment tax credit |
|
|
|
|
|
|
||
Unrecognized Canadian federal investment tax credits may be used to reduce future Canadian federal income tax and expire as follows:
F-19
|
|
$ |
|
|
2029 |
|
|
||
2030 |
|
|
|
|
2031 |
|
|
|
|
2032 |
|
|
|
|
2033 |
|
|
|
|
2034 |
|
|
|
|
2035 |
|
|
|
|
2036 |
|
|
|
|
2037 |
|
|
|
|
2038 |
|
|
|
|
2039 |
|
|
|
|
2040 |
|
|
|
|
2041 |
|
|
|
|
2042 |
|
|
|
|
2043 |
|
|
|
|
2044 |
|
|
|
|
|
|
|
|
|
13. Commitments and contingencies
Research and development contracts and contract research organizations agreements
The Company utilizes contract manufacturing organizations (“CMOs”) for the development and production of clinical materials and contract research organizations (“CROs”) to perform services related to its clinical trials. Pursuant to the agreements with these CMOs and CROs, the Company has either the right to terminate the agreements without penalties or under certain penalty conditions. As of March 31, 2024, the Company has
Raw krill oil supply contract
On October 25, 2019, the Company signed a supply agreement with Aker BioMarine Antarctic AS. (“AKBM”) to purchase raw krill oil product for a committed volume of commercial starting material for CaPre, one of the Company’s former drug candidates, for a total fixed value of $
F-20
Legal proceedings and disputes
In the ordinary course of business, the Company is at times subject to various legal proceedings and disputes. The Company assesses its liabilities and contingencies in connection with outstanding legal proceedings utilizing the latest information available. Where it is probable that the Company will incur a loss and the amount of the loss can be reasonably estimated, the Company records a liability in its consolidated financial statements. These legal contingencies may be adjusted to reflect any relevant developments. Where a loss is not probable or the amount of loss is not estimable, the Company does not accrue legal contingencies. While the outcome of legal proceedings is inherently uncertain, based on information currently available, management believes that it has established appropriate legal reserves. Any incremental liabilities arising from pending legal proceedings are not expected to have a material adverse effect on the Company’s financial position, results of operations, or cash flows. However, it is possible that the ultimate resolution of these matters, if unfavorable, may be material to the Company’s financial position, results of operations, or cash flows.
14. Restructuring Costs
On May 8, 2023, the Company communicated its decision to terminate a substantial amount of its workforce as part of a plan that intended to align the Company’s organizational and management cost structure to prioritize resources to GTX-104, thereby reducing losses to improve cash flow and extend available cash resources. The Company incurred $
F-21