424B4: Prospectus filed pursuant to Rule 424(b)(4)
Published on December 22, 2017
Table of Contents
Filed Pursuant to Rule 424(b)(4)
Registration No. 333-220755
PROSPECTUS

9,900,990 Common Shares
and
Warrants to Purchase up to 8,910,891 Common Shares
(8,910,891 Common Shares
Underlying the Warrants)
Acasti Pharma Inc. is offering 9,900,990 common shares, together with warrants to purchase up to 8,910,891 common shares. Each accompanying warrant is to purchase 0.90 of a common share. The common shares and warrants will be separately issued but will be purchased together in this offering. Each full warrant will have an exercise price of US$1.26 per share, will be exercisable upon issuance and will expire five years from the date on which such warrants were issued.
Our common shares are listed for trading on the NASDAQ Stock Market and the TSX Venture Exchange under the symbol ACST. On December 20, 2017, the closing price of our common shares on the NASDAQ Stock Market was US$1.18 per share and on the TSX Venture Exchange was $1.52 per share. There is no established trading market for the warrants. We do not intend to apply for any listing of the warrants on the NASDAQ Stock Market or the TSX Venture Exchange or any other securities exchange or nationally recognized trading system.
We are an emerging growth company under the U.S. Jumpstart Our Business Startups Act of 2012.
Investing in our securities involves risks. See Risk Factors beginning on page 9 of this prospectus.
PRICE US$1.009 PER SHARE AND US$0.001 PER WARRANT
| Per Share and Warrant(1) |
Total | |||||||
| Price to the public |
US$ | 1.01 | US$ | 10,000,000 | ||||
| Underwriting discount(1)(2) |
US$ | 0.0707 | US$ | 700,000 | ||||
| Proceeds to us (before expenses) |
US$ | 0.9393 | US$ | 9,300,000 | ||||
| (1) | The public offering price and underwriting discount correspond to a public offering price per share of US$1.009 and a public offering price per warrant of US$0.001. |
| (2) | We have also agreed to issue to the underwriters warrants to purchase common shares in an amount equal to 5% of the aggregate number of shares sold in this offering and to reimburse the underwriters for certain of their expenses. Please see Underwriting for a complete description of the compensation payable to the underwriters. |
We have granted the underwriters an option to purchase up to an additional 1,100,110 common shares and/or warrants to purchase up to an aggregate of 990,099 common shares at an exercise price of US$1.26 per share to cover over-allotments, if any. The underwriters can exercise this option at any time within 30 days after the date of this prospectus.
Neither the United States Securities and Exchange Commission nor any state securities commission has approved or disapproved of the securities or passed upon the accuracy or adequacy of this prospectus. Any representation to the contrary is a criminal offense.
The underwriters expect to deliver the common shares and the warrants against payment on or about December 27, 2017 which is the third business day following the date of pricing of the common shares and accompanying warrants (such settlement being referred to as T+3). Under Rule 15c6-1 of the Securities Exchange Act of 1934, as amended, trades in the secondary market generally are required to settle in two business days unless the parties to any trade expressly agree otherwise. See Underwriting.
| Benchmark | Dawson James Securities, Inc. |
The date of this prospectus is December 21, 2017.
Table of Contents
| 1 | ||||
| 2 | ||||
| 6 | ||||
| 8 | ||||
| 32 | ||||
| 35 | ||||
| 36 | ||||
| 37 | ||||
| 38 | ||||
| 40 | ||||
| 42 | ||||
| 66 | ||||
| MANAGEMENTS DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
67 | |||
| 86 | ||||
| 98 | ||||
| 100 | ||||
| 102 | ||||
| 104 | ||||
| 107 | ||||
| 108 | ||||
| 113 | ||||
| 125 | ||||
| 134 | ||||
| 136 | ||||
| 137 | ||||
| |
138 |
|
||
| 139 | ||||
| 140 | ||||
| F-1 |
Neither we nor the underwriters have authorized anyone to provide information different from that contained in this prospectus, any amendment or supplement to this prospectus or in any free writing prospectus prepared by us or on our behalf. Neither we nor the underwriters take any responsibility for, and can provide no assurance as to the reliability of, any information other than the information in this prospectus, any amendment or supplement to this prospectus, and any free writing prospectus prepared by us or on our behalf. Neither the delivery of this prospectus nor the sale of our securities means that information contained in this prospectus is correct after the date of this prospectus. This prospectus is not an offer to sell or the solicitation of an offer to buy our securities in any circumstances under which such offer or solicitation is unlawful.
We are offering to sell, and seeking offers to buy, securities only in jurisdictions where offers and sales are permitted. Neither we nor any of the underwriters have done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. You are required to inform yourselves about and to observe any restrictions relating to this offering and the distribution of this prospectus. The offered securities have not been and will not be qualified for distribution pursuant to a prospectus filed with the securities regulatory authorities in any of the provinces or territories of Canada and may not be offered or sold in Canada except pursuant to an exemption from the prospectus requirements of applicable Canadian securities laws.
Table of Contents
Except as otherwise indicated, references to Acasti, the Corporation, we, us, it, its, our or similar terms refer to Acasti Pharma Inc.
This prospectus contains company names, product names, trade names, trademarks and service marks of Acasti, Neptune Technologies & Bioressources Inc. and other organizations, all of which are the property of their respective owners. We own or have rights to trademarks, service marks or trade names that we use in connection with the operation of our business. In addition, our name, logo and website names and addresses are our service marks or trademarks. CaPre® and the phrase BREAKING DOWN THE WALLS OF CHOLESTEROL are our registered trademarks. The other trademarks, trade names and service marks appearing in this prospectus are the property of their respective owners. Solely for convenience, the trademarks, service marks, tradenames and copyrights referred to in this prospectus are listed without the ©, ® and TM symbols, but we will assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensors to these trademarks, service marks and tradenames.
Our financial information contained herein are presented in Canadian dollars. All references in this prospectus to dollars, CDN$ and $ refer to Canadian dollars, and references to US$ refer to United States dollars. Potential purchasers should be aware that foreign exchange rate fluctuations are likely to occur from time to time and that we do not make any representation with respect to future currency values. Investors should consult their own advisors with respect to the potential risk of currency fluctuations.
All financial information derived from our financial statements contained in this prospectus is presented in accordance with International Financial Reporting Standards, or IFRS, as issued by the International Accounting Standards Board, or IASB. We use multiple financial measures for the review of our operating performance. These measures are generally IFRS financial measures, but one adjusted financial measure, Non-IFRS operating loss (adding to net loss, finance expenses, depreciation and amortization and impairment loss, change in fair value of derivative warrant liabilities, stock-based compensation and subtracting finance income and deferred income tax recovery), is also used to assess our operating performance. We use this measure, in addition to the IFRS financial measures, for the purposes of evaluating our historical and prospective financial performance, as well as our performance relative to competitors and to plan and forecast future periods as well as to make operational and strategic decisions. We believe that providing this non-IFRS information to investors, in addition to IFRS measures, allows them to see our results through the eyes of our management, and to better understand our historical and future financial performance. See Managements Discussion and Analysis of Financial Condition and Results of OperationsCaution Regarding Non-IFRS Financial Measures.
Unless otherwise indicated, market data and certain industry data and forecasts included in this prospectus concerning our industry and the markets in which we operate or seek to operate were obtained from internal company surveys, market research, publicly available information, reports of governmental agencies and industry publications and surveys. We have relied upon industry publications as our primary sources for third-party industry data and forecasts. Industry surveys, publications and forecasts generally state that the information contained therein has been obtained from sources believed to be reliable, but that the accuracy and completeness of such information is not guaranteed. We have not independently verified any of the data from third-party sources, nor have we ascertained the underlying economic assumptions relied upon therein. Similarly, internal surveys, industry forecasts and market research, which we believe to be reliable based upon managements knowledge of the industry, have not been independently verified. By their nature, forecasts are particularly subject to change or inaccuracies, especially over long periods of time. In addition, we do not know what assumptions regarding general economic growth were used in preparing the forecasts cited in this prospectus. While we are not aware of any misstatements regarding the industry data presented herein, our estimates involve risks and uncertainties and are subject to change based on various factors, including those discussed under Cautionary Statement Regarding Forward-Looking Statements and Risk Factors in this prospectus. While we believe our internal business research is reliable and market definitions are appropriate, neither such research nor definitions have been verified by any independent source.
- 1 -
Table of Contents
This summary highlights information contained elsewhere in this prospectus. This summary may not contain all of the information that you should consider before deciding to invest in our securities. You should carefully read this entire prospectus, including the sections of this prospectus entitled Risk Factors and Managements Discussion and Analysis of Financial Condition and Results of Operations, and our financial statements and related notes, before investing in our securities.
Our Company
We are a biopharmaceutical innovator focused on the research, development and commercialization of prescription drugs using omega 3, or OM3, fatty acids derived from krill oil. OM3 fatty acids have extensive clinical evidence of safety and efficacy in lowering triglycerides, or TGs, in patients with hypertriglyceridemia, or HTG. Our lead product candidate is CaPre, an OM3 phospholipid, which we are developing initially for the treatment of severe HTG, a condition characterized by very high levels of TGs in the bloodstream (³ 500 mg/dL). Market research commissioned by us suggests there is a significant unmet medical need for an effective, safe and well-absorbing OM3 therapeutic that demonstrates a positive impact on the major blood lipids associated with cardiovascular disease risk. We believe that, if supported by our Phase 3 program in the United States, which we initiated during the second half of 2017 and for which we plan to start clinical site activation by the end of 2017, CaPre will address this unmet medical need. We also believe the potential exists to expand CaPres initial indication to patients with high TGs (blood levels between 200 499 mg/dL), although at least one additional clinical trial would likely be required to expand CaPres indication to this segment. We may seek to identify new potential indications for CaPre that may be appropriate for future studies and pipeline expansion. In addition, we may also seek to in-license other cardiometabolic drug candidates for drug development and commercialization.
In four clinical trials conducted to date, we saw the following beneficial effects with CaPre, and we are seeking to demonstrate similar results in our Phase 3 program:
| | significant reduction of TGs and non-high-density lipoprotein cholesterol (non-HDL-C) levels in the blood of patients with mildly elevated to severe HTG; |
| | no deleterious effect on low-density lipoprotein cholesterol, or LDL-C, or bad cholesterol, with the potential to reduce LDL-C; |
| | potential to increase high-density lipoprotein cholesterol, or HDL-C, or good cholesterol; |
| | good bioavailability (absorption by the body), even under fasting conditions; |
| | no significant food effect (meaning minimal difference in absorption) when taken with low-fat or high-fat meals; and |
| | an overall safety profile similar to that demonstrated by currently marketed OM3s. |
We believe that these features could set CaPre apart from current FDA-approved OM3 treatment options, and could give us a significant clinical and marketing advantage.
CaPre is a krill oil-derived mixture containing polyunsaturated fatty acids, or PUFAs, primarily composed of OM3 fatty acids, principally eicosapentaenoic acid, or EPA, and docosahexaenoic acid, or DHA, present as a combination of phospholipid esters and free fatty acids. EPA and DHA are well known to be beneficial for human health, and according to numerous recent clinical studies, may promote healthy heart, brain and visual function, and may also contribute to reducing inflammation and blood TGs. Krill is a natural source of phospholipids and OM3 fatty acids. The EPA and DHA contained in CaPre are delivered as a combination of
- 2 -
Table of Contents
OM3s as free fatty acids and OM3s bound to phospholipid esters, allowing these PUFAs to reach the small intestine where they undergo rapid absorption and transformation into complex fat molecules that are required for lipid transport in the bloodstream. We believe that EPA and DHA are more efficiently transported by phospholipids sourced from krill oil than the EPA and DHA contained in fish oil that are transported either by TGs (as in dietary supplements) or as ethyl esters in other prescription OM3 drugs (such as LOVAZA and VASCEPA), which must then undergo additional digestion before they are ready for transport into the bloodstream. The digestion and absorption of OM3 ethyl ester drugs requires a particular enzymatic process that is highly dependent on the fat content of a meal the higher the fat content, the better the OM3 ethyl ester absorption. High fat content meals are not recommended in patients with HTG. We believe that CaPres superior absorption profile could represent a significant clinical advantage, since taking it with a low-fat meal represents a more realistic regimen for patients with HTG who must follow a restricted low-fat diet.
CaPre is intended to be used as a therapy combined with positive lifestyle changes, such as a healthy diet and exercise, and can be administered either alone or with other drug treatment regimens such as statins (a class of drug used to reduce LDL-C). CaPre is intended to be taken orally once or twice per day in capsule form.
Key elements of our business and commercialization strategy include initially obtaining regulatory approval for CaPre in the United States for severe HTG. Currently, we do not have dedicated in-house sales and marketing personnel, and we are evaluating several alternative go-to-market strategies for commercializing CaPre in the United States, including through strategic partnerships as well as building our own sales and marketing organization. Our preferred strategy outside the United States is to commercialize CaPre through regional or country-specific strategic partnerships, and to potentially seek support and funding from each partner for clinical development, registration and commercialization activities. We believe that a late development-stage and differentiated drug candidate like CaPre could be attractive to various global, regional or specialty pharmaceutical companies, and we are taking a targeted approach to partnering and licensing in various geographies.
Our key commercialization goals include:
| | completing our Phase 3 program and, assuming the results are positive, filing a new drug application, or NDA, to obtain regulatory approval for CaPre in the United States, initially for the treatment of severe HTG, with the potential to afterwards expand CaPres indication to the treatment of high TGs (although at least one additional clinical trial would likely be required to expand CaPres indication to this segment); |
| | continuing to strengthen our patent portfolio and other intellectual property rights; |
| | continuing to evaluate and determine the optimal strategic approach for commercializing CaPre in the United States; and |
| | pursuing strategic opportunities outside of the United States, such as licensing or similar transactions, joint ventures, partnerships, strategic alliances or alternative financing transactions, to provide development capital, market access and other strategic sources of capital for us. |
In addition to completing our Phase 3 program, we expect that additional time and capital will be required to complete the filing of an NDA to obtain FDA pre-market approval for CaPre in the United States, and to complete business development collaborations, marketing and other pre-commercialization activities before reaching the commercial launch of CaPre.
Summary Risk Factors
Investing in our securities involves a high degree of risk. You should carefully consider the risks described in Risk Factors before making a decision to invest in our securities. If any of these risks actually occur,
- 3 -
Table of Contents
our business, financial condition and results of operations would likely be materially adversely affected. In such a case, the trading price of our securities would likely decline and you may lose part or all of your investment. Below is a summary of some of the principal risks we face:
| | we may not be able to maintain our operations and advance our research and development of CaPre without additional funding; |
| | if we encounter difficulties enrolling patients in our Phase 3 program, our development activities for CaPre could be delayed or otherwise adversely affected; |
| | our prospects currently depend entirely on the success of CaPre, which is still in clinical development, and we may not be able to generate revenues from CaPre; |
| | we may not be able to obtain required regulatory approvals for CaPre; |
| | we may not achieve our publicly announced milestones on time, or at all; |
| | if outcome studies being conducted by two of our competitors testing the impact of OM3s as an add-on to statin therapy on treating patients with high TGs are negative, there could also be an adverse impact for CaPre; |
| | recent and future legal developments could make it more difficult and costly for us to obtain regulatory approvals for CaPre and negatively affect the prices we may charge; |
| | we may not be able to compete effectively against our competitors pharmaceutical products; |
| | we may never become profitable or be able to sustain profitability; |
| | we may not be able to attain our targeted cost of goods sold, and levels of insurance reimbursement for CaPre may not be commercially viable in all global markets; |
| | we currently have no marketing and sales personnel and, as a company, no experience in marketing products. If we are unable to establish marketing and sales capabilities or enter into agreements with a strategic partner to market and sell CaPre, we may not be able to generate revenue; |
| | we may not reach a definitive agreement in connection with our recently announced non-binding term sheet with a leading China-based pharmaceutical company that would grant the China-based company an exclusive license to commercialize CaPre in certain Asian countries; |
| | even if we receive regulatory approval for CaPre, it may just be for a limited indication; |
| | we rely on third parties to conduct our clinical trials for CaPre; |
| | we rely on third parties to manufacture, produce and supply CaPre and may be adversely affected if those third parties are unable or unwilling to fulfill their obligations, including complying with FDA requirements; |
| | in the past, Neptune Technologies & Bioressources Inc., or Neptune, supplied us with the krill oil to produce CaPre for our clinical programs, including the krill oil projected to be needed for our Phase 3 program, and we will need to source alternative supplies of krill oil for future commercial supplies in light of Neptunes recent announcement to discontinue krill oil production; |
| | it is difficult and costly to protect our intellectual property rights; |
| | we rely on a sublicense granted to us by Neptune through its license with Aker BioMarine Antarctic AS in order for us to have freedom-to-operate for CaPre and we may not be able to manufacture and market CaPre if our sublicense is terminated; |
| | CaPre may infringe the intellectual property rights of others, which could increase our costs and delay or prevent CaPres development and commercialization efforts; and |
| | there is a significant risk that we may be characterized as a passive foreign investment company for U.S. federal income tax purposes. |
- 4 -
Table of Contents
Implications of Being an Emerging Growth Company and a Foreign Private Issuer
As a company with less than US$1.07 billion in revenue during our most recently completed fiscal year, we qualify as an emerging growth company as defined in Section 2(a) of the Securities Act of 1933, as amended (the Securities Act) as modified by the Jumpstart our Business Startups Act of 2012 (the JOBS Act). As an emerging growth company, we may take advantage of specified reduced disclosure and other requirements that are otherwise applicable generally to public companies that are not emerging growth companies. These provisions include an exemption from the auditor attestation requirement in the assessment of our internal control over financial reporting.
We may take advantage of these exemptions until we are no longer an emerging growth company. We will cease to be an emerging growth company on the last day of our fiscal year following the fifth anniversary of the first sale of our common shares pursuant to an effective registration statement. We also would cease to be an emerging growth company if we have US$1.07 billion or more in annual revenues as of the end of our fiscal year, more than US$700 million in market value of our shares held by non-affiliates as of the end of our second fiscal quarter, or we issue more than US$1.0 billion of non-convertible debt securities over a three-year period. We may choose to take advantage of some but not all of these reduced disclosure obligations. If we do, the information that we provide shareholders may be different than you might get from other public companies in which you hold shares.
We report under the Securities Exchange Act of 1934, as amended (the Exchange Act) as a non-U.S. company with foreign private issuer status. As a foreign private issuer, we may take advantage of certain provisions in the NASDAQ Listing Rules that allow us to follow Canadian law for certain corporate governance matters. Even after we no longer qualify as an emerging growth company, as long as we qualify as a foreign private issuer under the Exchange Act, we will be exempt from certain provisions of the Exchange Act that are applicable to U.S. domestic public companies, including:
| | the sections of the Exchange Act regulating the solicitation of proxies, consents or authorizations in respect of a security registered under the Exchange Act; |
| | the sections of the Exchange Act requiring insiders to file public reports of their stock ownership and trading activities and liability for insiders who profit from trades made in a short period of time; |
| | the rules under the Exchange Act requiring the filing with the U.S. Securities and Exchange Commission of quarterly reports on Form 10-Q containing unaudited financial and other specified information, or current reports on Form 8-K, upon the occurrence of specified significant events; |
| | the sections of the Exchange Act requiring U.S. GAAP financial statements (rather than financial statements pursuant to IFRS as issued by the IASB used by us); and |
| | Regulation Fair Disclosure, or Regulation FD, which regulates selective disclosures of material information by issuers. |
Both foreign private issuers and emerging growth companies are also exempt from certain more stringent executive compensation disclosure rules. Even if we no longer qualify as an emerging growth company, but remain a foreign private issuer, we will continue to be exempt from the more stringent compensation disclosures required of companies that are neither an emerging growth company nor a foreign private issuer.
Corporate Information
We were incorporated on February 1, 2002 under Part 1A of the Companies Act (Québec) under the name 9113-0310 Québec Inc. On August 7, 2008, pursuant to a Certificate of Amendment, we changed our name to Acasti Pharma Inc. and on February 14, 2011, the Business Corporations Act (Québec) came into effect and replaced the Companies Act (Québec). We are now governed by the Business Corporations Act (Québec), or the QBCA.
Our principal executive offices are currently located at 545 Promenade du Centropolis, Suite 100, Laval, Québec, Canada H7T 0A3. Our telephone number is (450) 686-4555.
- 5 -
Table of Contents
| Common shares being offered |
9,900,990 common shares (or 8,910,891 common shares if the underwriters exercise their option to purchase additional shares in full). |
| Common shares to be outstanding immediately after this offering |
24,815,648 common shares (or 25,915,758 common shares if the underwriters exercise their option to purchase additional shares in full). |
| Warrants |
Each common share offered is being sold together with a warrant to purchase 0.90 of a common share. Each full warrant will be exercisable at an exercise price of US$1.26 per share. The warrants are exercisable at any time for a period of five years from the date on which such warrants were issued. This prospectus also relates to the offering of the common shares issuable upon exercise of the warrants. |
| Over-allotment option |
We have granted the underwriters an option for a period of up to 30 days from the date of this prospectus to purchase up to an additional 1,100,110 common shares and/or warrants to purchase up to an aggregate of 990,099 common shares at an exercise price of US$1.26 per share in any combinations thereof, to cover over-allotments, if any, at the public offering price less the underwriting discounts and commissions. |
| Because the warrants are not listed on a national securities exchange or other nationally recognized trading market, the underwriters will be unable to satisfy any overallotment of shares and warrants without exercising the underwriters overallotment option with respect to the warrants. As a result, the underwriters will exercise their overallotment option for all of the warrants which are over-allotted, if any, at the time of the initial offering of the shares and the warrants. However, because our common stock is publicly traded, the underwriters may satisfy some or all of the overallotment of shares of our common stock, if any, by purchasing shares in the open market and will have no obligation to exercise the overallotment option with respect to our common stock. |
| NASDAQ Stock Market and TSXV symbol |
ACST |
| Use of proceeds |
We estimate that we will receive net proceeds from this offering of approximately US$8.6 million, or US$9.6 million if the underwriters exercise in full their option to purchase additional common shares and warrants, after deducting underwriting discounts and commissions and estimated offering expenses. |
| We currently intend to use the net proceeds of this offering, together with our cash on hand, for the further development of CaPre, including clinical site activation, progression of patient enrollment and production of clinical materials (both CaPre and placebo) for our Phase 3 program; expansion of business development activities; working capital; and other general corporate purposes. |
- 6 -
Table of Contents
| Dividend policy |
We have not declared any dividends since our inception and do not anticipate that we will do so in the foreseeable future. We currently intend to retain future earnings, if any, to finance the development of our business. Any future payment of dividends or distributions will be determined by our board of directors on the basis of our earnings, financial requirements and other relevant factors. |
| Risk factors |
Investing in our securities involves a high degree of risk and purchasers of our securities may lose part or all of their investment. See Risk Factors for a discussion of factors you should carefully consider before deciding to invest in our securities. |
The number of our common shares to be outstanding after this offering is based on 14,914,658 of our common shares outstanding as of December 20, 2017. The number of common shares to be outstanding after this offering excludes the following:
| | 2,395,788 common shares issuable upon the exercise of options issued to our directors, officers and employees, at a weighted-average exercise price of $1.82 per common share; |
| | 1,052,630 common shares issuable upon conversion of debentures at an exercise price of $1.90 per common share; |
| | 1,840,000 common shares issuable upon the exercise of warrants at a weighted-average exercise price of US$15.00 per common share; |
| | 161,654 common shares issuable upon the exercise of warrants at a weighted-average exercise price of $13.30 per common share; |
| | 1,904,034 common shares issuable upon the exercise of warrants at an exercise price of $2.15 per common share; |
| | 117,496 common shares issuable upon the exercise of broker warrants at an exercise price of $2.15 per common share; |
| | 495,050 common shares issuable upon the exercise of underwriter warrants at an exercise price of US$1.26 per common share issued in connection with this offering; and |
| | 8,910,891 common shares issuable upon the exercise of warrants to be issued to investors in connection with this offering at an exercise price of US$1.26 per common share. |
Additionally, except as otherwise indicated herein, all information in this prospectus assumes no exercise of the underwriters option to purchase additional common shares and warrants.
- 7 -
Table of Contents
An investment in our securities involves a high degree of risk and should be considered speculative. An investment in our securities should only be undertaken by those persons who can afford the total loss of their investment. You should carefully consider the risks and uncertainties described below, as well as other information contained in this prospectus. Additional risks and uncertainties not presently known to us or that we believe to be immaterial may also adversely affect our business. If any of these risks actually occur, our business, financial condition, prospects, results of operations or cash flow could be materially and adversely affected and you could lose all or a part of the value of your investment. Certain statements below are forward-looking statements. See Cautionary Statement Regarding Forward-Looking Statements.
Risks Facing Our Business and Industry
We may not be able to maintain our operations and advance our research and development of CaPre without additional funding.
We have incurred operating losses and negative cash flows from operations since our inception. To date, we have financed our operations through public offerings and private placements of securities, proceeds from exercises of warrants, rights and options, and receipt of research tax credits and research grant programs. Our cash and cash equivalents (including restricted investments) were $5.3 million as of September 30, 2017, $9.8 million as of March 31, 2017 and $12.5 million as of February 29, 2016. Our current assets as at September 30, 2017 are projected to be significantly less than needed to support our current liabilities as at September 30, 2017 when combined with our projected level of expenses for the next twelve months, including initiation of, and enrollment of patients in, our Phase 3 program for CaPre. Our positive working capital balance has declined during the current fiscal year and is expected to continue to decline until we raise additional funds or find a strategic partner. We will also require substantial additional funds to conduct further research and development and our Phase 3 program, obtain regulatory approvals and commercialize CaPre. In addition to completing nonclinical and clinical trials, we expect that additional time and capital will be required by us to file an NDA to obtain FDA approval for CaPre in the United States and to complete marketing and other pre-commercialization activities. We will also most likely require additional capital to fund our daily operating needs. Based on a conservative estimate, we believe that the net proceeds of this offering, together with our existing cash and cash equivalents, will enable us to fund our operating expenses and capital expenditure requirements through May 2018. To achieve our business plan, we will need to raise the necessary capital primarily through additional securities offerings and strategic alliances in the near term. We have no committed source of additional capital from Neptune, which currently owns approximately 34% of our common shares, or any other party, and if we are unable to raise additional capital in sufficient amounts or on terms acceptable to us, we may have to significantly delay, scale back or discontinue our development or commercialization of CaPre or our other research and development initiatives. Funding needs could also force us to seek strategic partners for CaPre at an earlier stage than we desire or on terms that are less favorable to us or force us to relinquish or license our rights to CaPre on unfavorable terms or in markets where we would prefer to pursue development or commercialization ourselves. Additional funding from third parties may not be available on acceptable terms or at all to enable us to continue and complete our research and development of CaPre.
We recently entered into a non-binding term sheet with a leading China-based pharmaceutical company. Completion of the transaction is subject to further negotiation and execution of a definitive agreement, which once signed would grant an exclusive license to the Chinese pharmaceutical company to commercialize CaPre in certain Asian countries, including China. If a definitive agreement is reached and signed, the term sheet contemplates that we would receive an upfront payment of US$8 million upon signing, plus potential regulatory and commercial milestone payments in excess of US$125 million, and tiered double-digit royalties on net sales. The term sheet contemplates that the term of the license, including the period during which milestone payments, if any, could be achieved, would be the later of (i) the fifth anniversary of the last-to-expire patent or (ii) 2035, and the license would be automatically renewable for one renewal term of ten years. The term sheet is preliminary and non-binding at this stage and the license, upfront payment, possible milestone payments and royalties contemplated by it will only become operative if definitive documents are executed. It is possible that no definitive agreement will be reached, or, if a definitive agreement is reached, that its terms and conditions
- 8 -
Table of Contents
may differ from those described above. If we do enter into definitive documents, the near term timing of the next steps in the advancement of our research and development of CaPre could be affected, depending on whether our development of CaPre in those Asian countries would be pursued under a separate clinical program or as part of our existing Phase 3 clinical program.
If we do not raise additional funds or find one or more strategic partners, we may not be able to realize our assets and discharge our liabilities in the normal course of business. As a result, there exists a material uncertainty that casts substantial doubt about our ability to continue as a going concern and, therefore, realize our assets and discharge our liabilities in the normal course of business. Our financial statements have been prepared on a going-concern basis, which assumes we will continue our operations in the foreseeable future and will be able to realize our assets and discharge our liabilities and commitments in the ordinary course of business. If we are unable to continue as a going concern, material write-downs to the carrying value of our assets, including intangible assets, could be required. If we fail to obtain additional financing, we may not be able to continue as a going concern.
If we encounter difficulties enrolling patients in our Phase 3 program, our development activities for CaPre could be delayed or otherwise adversely affected.
We may experience difficulties in patient enrollment in our clinical trials, including our Phase 3 program for CaPre, for a variety of reasons. Timely completion of our clinical trials in accordance with their protocols depends, among other things, on our ability to enroll a sufficient number of patients who remain in the trial until its conclusion. The enrollment of patients depends on many factors, including:
| | the number of clinical trials for other product candidates in the same therapeutic area that are currently in clinical development, and our ability to compete with those trials for patients and clinical trial sites; |
| | patient eligibility criteria defined in the protocol; |
| | the size of the patient population; |
| | the risk that disease progression will result in death before the patient can enroll in clinical trials or before the completion of any clinical trials in which the patient is enrolled; |
| | the proximity and availability of clinical trial sites for prospective patients; |
| | the design of the trial; |
| | our ability to recruit clinical trial investigators with the appropriate competencies and experience; |
| | our ability to obtain and maintain patient consents; and |
| | the risk that patients enrolled in clinical trials will drop out of the trials before completion. |
Our Phase 3 program for CaPre may compete with other clinical trials for product candidates that are in the same therapeutic areas as CaPre. This competition could reduce the number and types of patients and qualified clinical investigators available to us, because some patients who might have opted to enroll in our Phase 3 program may instead opt to enroll in a trial being conducted by one of our competitors or a clinical trial site may not allow us to conduct our clinical program at that site if competing trials are already being conducted there. We may also encounter difficulties finding adequate clinical trial sites at which to conduct our Phase 3 program. Delays in patient enrollment may result in increased costs or may affect the timing or outcome of our Phase 3 program, which could impair or prevent its completion and adversely affect our ability to advance the development of CaPre.
Our prospects currently depend entirely on the success of CaPre, which is still in clinical development, and we may not be able to generate revenues from CaPre.
We have no prescription drug products that have been reviewed or approved by the FDA, Health Canada or any similar regulatory authority. Our only prescription drug candidate is CaPre, for which we have not yet filed an
- 9 -
Table of Contents
NDA, and for which we must conduct a Phase 3 program, undergo further development activities and seek and receive regulatory approval prior to commercial launch, which we do not anticipate will occur until the second half of 2021 at the earliest. We have invested significant effort and financial resources in researching and developing CaPre. Further development of CaPre will require substantial investment, access to sufficient commercial manufacturing capacity and significant marketing efforts before we can generate any revenue from sales of CaPre, if it is ever approved for commercialization.
We do not have any other prescription drug candidates in development and so our business prospects currently depend entirely on the successful development, regulatory approval and commercialization of CaPre, which may never occur. Most prescription drug candidates never reach the clinical development stage and even those that do reach clinical development have only a small chance of successfully completing clinical development and gaining regulatory approval. If we are unable to successfully commercialize CaPre, we may never generate meaningful revenues. In addition, if CaPre reaches commercialization and there is low market demand for CaPre or the market for CaPre develops less rapidly than we anticipate, we may not have the ability to shift our resources to the development of alternative products.
We may not be able to obtain required regulatory approvals for CaPre.
We have limited experience in conducting and managing the clinical trials necessary to obtain regulatory approvals, including approval by the FDA and, as a company, we have no experience in obtaining approval of any product candidates. The research, testing, manufacturing, labeling, packaging, storage, sale, marketing, pricing, export, import and distribution of prescription drug products are subject to extensive regulation by the FDA and other regulatory authorities in the United States and other countries and those regulations differ from country to country. We are not permitted to market CaPre in the United States until we receive approval of an NDA from the FDA and similar restrictions apply in other countries. In the United States, the FDA generally requires the completion of preclinical testing and clinical trials of each drug to establish its safety and efficacy and extensive pharmaceutical development and manufacturing controls to ensure its quality before an NDA is approved. Regulatory authorities in other jurisdictions impose similar requirements. Of the large number of drugs in development, only a small percentage result in the submission of an NDA to the FDA and even fewer are approved for commercialization. To date, we have not submitted an NDA for CaPre to the FDA or comparable applications to other regulatory authorities.
Our receipt of required regulatory approvals for CaPre is uncertain and subject to a number of risks, including:
| | the FDA or comparable foreign regulatory authorities or independent institutional review boards, or IRBs, may disagree with the design or implementation of our clinical trials; |
| | we may not be able to provide acceptable evidence of the safety and efficacy of CaPre; |
| | the results of our clinical trials may not meet the level of statistical or clinical significance required by the FDA or other regulatory agencies for marketing approval; |
| | the dosing of CaPre in a particular clinical trial may not be at an optimal level; |
| | patients in our clinical trials may suffer adverse effects for reasons that may or may not be related to CaPre; |
| | we may be unable to demonstrate that CaPres clinical and other benefits outweigh its safety risks; |
| | the data collected from our clinical trials may not be sufficient to support the submission of an NDA for CaPre or to obtain regulatory approval for CaPre in the United States or elsewhere; |
| | the FDA or comparable foreign regulatory authorities may not approve the manufacturing processes or facilities of third party manufacturers with which we contract for clinical and commercial supplies of CaPre; and |
| | the law or regulations or approval policies of the FDA or comparable foreign regulatory authorities may significantly change in a manner rendering our clinical data insufficient for approval. |
- 10 -
Table of Contents
The FDA and other similar regulators have substantial discretion in the approval process and may refuse to accept our application or may decide that our data is insufficient for approval and require additional clinical trials, or preclinical or other studies for CaPre. If regulatory approval for CaPre is obtained in one jurisdiction, that does not necessarily mean that CaPre will receive regulatory approval in all jurisdictions in which we seek approval. If we fail to obtain approval for CaPre in one or more jurisdictions, our ability to obtain approval in a different jurisdiction may be negatively affected.
We may not achieve our publicly announced milestones on time, or at all.
From time to time, we may publicly announce the timing of certain events we expect to occur, such as the anticipated timing of results from our clinical trials. These statements are forward-looking and are based on the best estimate of management at the time relating to the occurrence of the events. However, the actual timing of these events may differ from what has been publicly disclosed. The timing of events such as completion of a clinical trial, discovery of a new product candidate, filing of an application to obtain regulatory approval, beginning of commercialization of products, or announcement of additional clinical trials for a product candidate may ultimately vary from what is publicly disclosed. For example, we cannot provide assurances that we will conduct our Phase 3 clinical trials for CaPre, that we will make regulatory submissions or receive regulatory approvals as planned, or that we will be able to adhere to plans for the scale-up of manufacturing and launch of CaPre. These variations in timing may occur as a result of different events, including the nature of the results obtained during a clinical trial or during a research phase, problems with a supplier or a distribution partner or any other event having the effect of delaying the publicly announced timeline. We undertake no obligation to update or revise any forward-looking information, whether as a result of new information, future events or otherwise, except as otherwise required by law. Any variation in the timing of previously-announced milestones could have a material adverse effect on our business, financial condition or operating results and the trading price of our common shares.
If outcome studies being conducted by two of our competitors testing the impact of OM3 on treating patients with high TGs are negative, there could also be an adverse impact for CaPre.
We are currently awaiting outcome study data from two of our competitors that are testing the effects of OM3 on patients with high TGs. These cardiovascular outcome studies are expected to report in mid-2018 (the REDUCE-IT trial sponsored by Amarin) and 2019 (the STRENGTH trial sponsored by AstraZeneca). If those studies show that OM3 therapeutic drugs effectively treat patients with high TGs and improve cardiovascular, morbidity and mortality outcomes, we believe that the potential to expand CaPres indication in the future to include the treatment of high TGs would be significantly advanced. Conversely, if outcome study data from one or both of those competitors is negative, or if one or both clinical trials fail to be completed, our potential target market for CaPre could be limited solely to patients with severe HTG and our ability to realize greater market potential of CaPre could be harmed.
Reimbursement decisions by third-party payors may have an adverse effect on pricing and market acceptance. If there is not sufficient reimbursement for CaPre, it is less likely that it will be widely used.
Even if CaPre is approved for sale by the appropriate regulatory authorities, market acceptance and sales of CaPre will depend on reimbursement policies and may be affected by future healthcare reform measures. Government authorities and third-party payors, such as private health insurers and health maintenance organizations, decide which drugs they will reimburse and establish payment levels. We cannot be certain that reimbursement will be available for CaPre. If reimbursement is not available or is available on a limited basis, we may not be able to successfully commercialize CaPre.
There may be significant delays in obtaining coverage and reimbursement for newly-approved drugs, and coverage may be more limited than the purposes for which the drug is approved by the FDA or other regulatory authorities. Moreover, eligibility for coverage and reimbursement does not imply that a drug will be paid for in all cases or at a rate that covers our costs, including research, development, manufacture, sale and distribution expenses. Interim
- 11 -
Table of Contents
reimbursement levels for new drugs, if applicable, may also be insufficient to cover our costs and may not be made permanent. Reimbursement rates may vary according to the use of a drug and the clinical setting in which it is used, may be based on reimbursement levels already set for lower cost drugs and may be incorporated into existing payments for other services. Net prices for drugs may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors and by any future relaxation of laws that presently restrict imports of drugs from countries where they may be sold at lower prices than in the United States. Our inability to promptly obtain coverage and profitable payment rates from both government-funded and private payors for CaPre could have a material adverse effect on our operating results and our overall financial condition.
Recent and future legal developments could make it more difficult and costly for us to obtain regulatory approvals for CaPre and negatively affect the prices we may charge.
In the United States and elsewhere, recent and proposed legal and regulatory changes to healthcare systems could prevent or delay our receipt of regulatory approval for CaPre, restrict or regulate our post-approval marketing activities, and adversely affect our ability to profitably sell CaPre. Proposals have also been made to expand post-approval requirements and to restrict sales and promotional activities for pharmaceutical products. We do not know whether additional legislative changes will be enacted, or whether the FDAs regulations, guidance or interpretations will be changed, or what impact any such changes will have, if any, on our ability to obtain regulatory approvals for CaPre. Further, the Centers for Medicare and Medicaid Services, or CMS, frequently changes product descriptors, coverage policies, product and service codes, payment methodologies and reimbursement values. Also, increased scrutiny by the U.S. Congress of the FDAs approval process could significantly delay or prevent our receipt of regulatory approval for CaPre and subject us to more stringent product labeling and post-marketing testing and other requirements.
In the United States, the Medicare Modernization Act, or the MMA, changed the way Medicare covers and pays for pharmaceutical products. The MMA expanded Medicare coverage for drug purchases by the elderly and introduced a new reimbursement methodology based on average sales prices for drugs. In addition, the MMA authorized Medicare Part D prescription drug plans to use formularies where they can limit the number of drugs that will be covered in any therapeutic class. As a result of the MMA and the expansion of federal coverage of drug products, we expect there will be additional pressure to contain and reduce healthcare costs. These healthcare cost reduction initiatives and other provisions of the MMA could decrease the coverage and price that we would receive for CaPre. While the MMA applies only to drug benefits for Medicare beneficiaries, private health insurance companies often follow Medicare coverage policy and payment limitations in setting their own reimbursement rates, and any reduction in reimbursement that results from the MMA may result in a similar reduction in payments from private health insurance companies.
The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act (the Health Care Reform Law), has broadened access to health insurance, reduced or constrained the growth of healthcare spending, enhanced remedies against fraud and abuse, added new transparency requirements for the healthcare and health insurance industries, imposed new taxes and fees on the health industry and imposed additional health policy reforms. Provisions of the Health Care Reform Law affecting pharmaceutical companies include requirements to offer discounts on brand-name drugs to patients who fall within the Medicare Part D coverage gap, commonly referred to as the donut hole, and to pay an annual non-tax deductible fee to the federal government based on each companys market share of prior year total sales of branded products to certain federal healthcare programs, such as Medicare, Medicaid, Department of Veterans Affairs and Department of Defense.
Despite initiatives to invalidate the Health Care Reform Law, the U.S. Supreme Court has upheld key aspects of it. Due to the results of the recent presidential election, the Health Care Reform Law may be significantly changed and we do not know whether any such changes could have significant negative financial impacts on the development or potential profitability of CaPre. At this time, it remains unclear whether there will be any changes made to the Health Care Reform Law, whether to certain provisions or its entirety. The Health Care Reform Law or any replacement of it could continue to apply downward pressure on pharmaceutical pricing,
- 12 -
Table of Contents
especially under the Medicare program, and may also increase our regulatory burdens and operating costs. Additional federal healthcare reform measures could be adopted in the future limiting the amounts that federal and state governments will pay for healthcare products and services, which could negatively affect the value of CaPre and our ability to achieve profitability.
In Canada, most new patented drug prices are limited so that the cost of therapy is in the range of the cost of therapy for existing drugs sold in Canada used to treat the same disease. As a result:
| | prices of moderate and substantial improvement drugs and breakthrough drugs are also restricted by a variety of tests; |
| | existing patented drug prices cannot increase by more than the Canadian Consumer Price Index; and |
| | the Canadian prices of patented medicines can never be the highest in the world. |
If CaPre receives regulatory approval in Canada, restrictions on the price we can charge there for CaPre could reduce the value of CaPre and our ability to generate revenue and achieve profitability.
In many jurisdictions outside the United States, a product candidate must be approved for health care reimbursement before it can be approved for sale. In some cases, the price that we intend to charge for CaPre will also be subject to approval. If we fail to comply with the regulatory requirements in our target international markets or to receive required marketing approvals, our potential market for CaPre will be reduced and our ability to realize the full market potential for CaPre will be harmed.
We may not be able to compete effectively against our competitors pharmaceutical products.
The biotechnology and pharmaceutical industries are highly competitive. There are many pharmaceutical companies, biotechnology companies, public and private universities and research organizations actively engaged in the research and development of products that may be similar to CaPre. It is probable that the number of companies seeking to develop products and therapies similar to CaPre will increase. Many of these and other existing or potential competitors have substantially greater financial, technical and human resources than we do and may be better equipped to develop, manufacture and market products. These companies may develop and introduce products and processes competitive with or superior to CaPre. In addition, other technologies or products may be developed that have an entirely different approach or means of accomplishing the intended purposes of CaPre, which might render our technology and CaPre non-competitive or obsolete.
Our competitors in the United States and globally include large, well-established pharmaceutical companies, specialty pharmaceutical sales and marketing companies, and specialized cardiovascular treatment companies. GlaxoSmithKline plc, which currently sells LOVAZA, a prescription-only OM3 fatty acid indicated for patients with severe HTG, was approved by FDA in 2004 and has been on the market in the United States since 2005. Multiple generic versions of LOVAZA are now available in the United States. Amarin launched its prescription-only OM3 drug VASCEPA in 2013, and reached a market share of approximately 20% by the end of 2015. In addition, EPANOVA (OM3-carboxylic acids) capsules, a free fatty acid form of OM3 (comprised of 55% EPA and 20% DHA), is FDA-approved for patients with severe HTG. Omtryg, another OM3 fatty acid composition developed by Trygg Pharma AS, received FDA approval for severe HTG. Neither EPANOVA nor Omtryg have yet been commercially launched, but could launch at any time. Other large companies with products competing indirectly with CaPre include AbbVie, Inc., which currently sells Tricor and Trilipix for the treatment of severe HTG, and Niaspan, which is primarily used to raise HDL-C but is also used to lower TGs. Generic versions of Tricor, Trilipix and Niaspan are also now available in the United States. In addition, we are aware of a number of other pharmaceutical companies that are developing products that, if approved and marketed, would compete with CaPre.
Even if it receives regulatory approval, CaPre may need to demonstrate compelling comparative advantages in efficacy, convenience, tolerability and safety to be commercially successful. Other competitive factors, including generic drug competition, could force us to lower prices or could result in reduced sales of CaPre. In addition,
- 13 -
Table of Contents
new products developed by others could emerge as competitors to CaPre. If we are not able to compete effectively against our current and future competitors, our business will not grow and our financial condition and operations will suffer.
We may never become profitable or be able to sustain profitability.
We are a clinical-stage biopharmaceutical company with a limited operating history. The likelihood of the success of our business plan must be considered in light of the problems, expenses, difficulties, complications and delays frequently encountered when developing and expanding early-stage businesses and the regulatory and competitive environment in which we operate. Biopharmaceutical product development is a highly speculative undertaking, involves a substantial degree of risk and is a capital- intensive business. We expect to incur expenses without any meaningful corresponding revenues unless and until we are able to obtain regulatory approval for and begin selling CaPre in significant quantities. We filed our investigational new drug application, or IND, for CaPre in late 2013, which allowed us to initiate clinical development in the United States towards FDA approval for CaPre. To date, we have not generated any revenue from CaPre, and we may never be able to obtain regulatory approval for marketing CaPre in any indication. Even if we are able to commercialize CaPre, we may still not generate significant revenues or achieve profitability. Additionally, we may not be able to attain our targeted cost of goods sold, and levels of insurance reimbursement for CaPre may not be commercially viable in all global markets. We incurred net losses of $4.5 million for the three-month period ended September 30, 2017, $7.3 million for the six-month period ended September 30, 2017, $11.2 million for the thirteen month period ended March 31, 2017, and $6.3 million and $1.7 million for our fiscal years ended 2016 and 2015, respectively. As of September 30, 2017, we had an accumulated deficit of $58.2 million.
We expect that our expenses will increase significantly as we continue our Phase 3 clincial program for CaPre under the current indication and prepare to seek FDA approval for the commercial launch of CaPre. We also expect that our research and development expenses will continue to increase if we pursue FDA approval for CaPre for other indications. As a result, we expect to continue to incur substantial losses for the foreseeable future, and these losses may be increasing. We are uncertain about when or if we will be able to achieve or sustain profitability. If we fail to become and remain profitable, our ability to sustain our operations and to raise capital could be impaired and the price of our common shares could decline.
We currently have no marketing and sales personnel and, as a company, no experience in marketing products. If we are unable to establish marketing and sales capabilities or enter into agreements with a strategic partner to market and sell CaPre, we may not be able to generate revenue.
We currently have no sales, marketing or distribution personnel and, as a company, we have no experience in marketing products. If CaPre or another of our future product candidates is approved for commercialization, unless we find a strategic partner to assist us with sales, marketing and distribution, we will be required to develop in-house marketing and sales force capability, which would require significant capital expenditures, management resources and time. Also, we would have to compete with other biotechnology and pharmaceutical companies to recruit, hire, train and retain marketing and sales personnel. We face competition in our search for strategic partners to assist us with sales, marketing and distribution, and we may not be able to establish or maintain any such arrangements. If we do find strategic partners, any revenue we receive from CaPre would partly depend upon the efforts of each strategic partner, which may not be successful. We may have little or no control over the marketing and sales efforts by any strategic partner we find for CaPre and our revenue may be lower than if we had commercialized CaPre independently.
If we are not successful in attracting and retaining highly qualified personnel, we may not be able to successfully implement our business strategy.
Our ability to compete in the highly competitive pharmaceuticals industry largely depends upon our ability to attract and retain highly qualified managerial, scientific and medical personnel. Competition for skilled personnel in our market is intense and competition for experienced scientists and business personnel may limit our ability
- 14 -
Table of Contents
to hire and retain highly qualified personnel on acceptable terms. We are highly dependent on our management, scientific and medical personnel. Despite our efforts to retain valuable employees, members of our management, scientific and medical teams may terminate their employment with us on short notice or potentially without any notice at all. The loss of the services of any of our executive officers or other key employees could potentially harm our business, operating results or financial condition. Our success may also depend on our ability to attract, retain and motivate highly skilled junior, mid-level, and senior managers and scientific personnel. In addition, we do not maintain key person insurance policies on the lives of our executives or those of any of our other employees. Other pharmaceutical companies with which we compete for qualified personnel have greater financial and other resources, different risk profiles, and a longer history in the industry than we do. They also may provide more diverse opportunities and better chances for career advancement. Some of these characteristics may be more appealing to high-quality candidates than what we can offer. If we are unable to continue to attract and retain high-quality personnel, the rate and success at which we can develop and commercialize CaPre and any other future product candidates would be limited.
Neptune has significant influence over matters we put to a vote of our shareholders.
Neptune currently owns approximately 34% of our outstanding common shares. As a result, Neptune has significant influence with respect to all matters submitted to our shareholders for approval, such as the election and removal of directors, amendments to our articles of incorporation and by-laws and the approval of certain business combinations. This concentration of holdings may cause the market price of our common shares to decline, delay or prevent any acquisition, delay or discourage take-over attempts that shareholders may consider to be favorable, or make it more difficult or impossible for a third party to acquire control of us or effect a change in our board of directors and management. Any delay or prevention of a change of control transaction could deter potential acquirers or prevent the completion of a transaction in which our shareholders could receive a premium over the then current market price for our common shares.
Neptunes interests may not align with those of us or our other shareholders.
Neptunes interests may not in all cases be aligned with interests of us or our other shareholders. Neptune may have an interest in pursuing acquisitions, divestitures and other transactions that may ultimately be detrimental to our business and negatively affect the market price of our common shares.
Business disruptions could seriously harm our future revenue and financial condition and increase our costs and expenses.
Our operations, and those of our suppliers, third party manufacturers and other contractors and consultants could be subject to earthquakes, power shortages, telecommunications failures, water shortages, floods, hurricanes, typhoons, fires, extreme weather conditions, medical epidemics and other natural or man-made disasters or business interruptions, for which we are predominantly self- insured. The occurrence of any of these business disruptions could seriously harm our operations and financial condition and increase our costs and expenses. We rely on third-party manufacturers to manufacture CaPre. Our ability to obtain supplies of CaPre could be disrupted if the operations of our manufacturers and suppliers are affected by a man-made or natural disaster or other business interruption.
Even if we receive regulatory approval for CaPre, it may just be for a limited indication.
If we obtain regulatory approval for CaPre, we will only be permitted to market it for the indication approved by the FDA, and any such approval may put limits on the indicated uses or promotional claims we may make for it, or otherwise not permit labeling that sufficiently differentiates CaPre from competitive products with comparable therapeutic profiles. For example, while our initial objective is to seek regulatory approval for the treatment of severe HTG, afterwards obtaining approval for CaPre to address high TGs could greatly expand our potential market for CaPre. However, even if CaPre is approved for severe HTG, it may never be approved for the treatment of high TGs. In addition, any approval we receive for CaPre could contain significant use restrictions
- 15 -
Table of Contents
for specified age groups, warnings, precautions or contraindications, or may be subject to burdensome post-approval study or risk management requirements. If any regulatory approval for CaPre contains significant limits, we may not be able to obtain sufficient funding or generate meaningful revenue from CaPre or be able to continue developing, marketing or commercializing CaPre.
We may be unable to find successful strategic partnerships to develop and commercialize CaPre.
We have started seeking co-development, licensing and/or marketing partnership opportunities with third parties that we believe will complement or augment our development and commercialization efforts for CaPre. Pursuing partnership relationships is requiring us to incur non-recurring and other charges, and may require us to increase our near and long-term expenditures, issue securities that dilute our existing shareholders or disrupt our management and business. Entering into partnership relationships in certain countries could also delay the development of CaPre and our other future product candidates in those countries if we become dependent upon a strategic partner and that strategic partner does not prioritize the development of CaPre relative to its other development activities. In addition, we face significant competition in seeking strategic partners and the negotiation process is time-consuming and complex. We may not be successful in our efforts to establish a strategic partnership or other alternative arrangements for CaPre on our anticipated timeline, or at all, because CaPre may be deemed to be at too early of a stage of development for collaborative effort and third parties may not view CaPre as having the requisite potential to demonstrate safety and efficacy. Even if we do enter into strategic partnerships, those partnerships may not achieve our objectives.
We may be unable to develop alternative product candidates.
To date, we have not commercialized any prescription drug candidates and, other than CaPre, we do not have any compounds in clinical trials, nonclinical testing, lead optimization or lead identification stages. If we fail to obtain regulatory approval for and successfully commercialize CaPre as a treatment for severe HTG or any other indication, whether as a stand-alone therapy or in combination with other treatments, we would have to develop, acquire or license alternative product candidates or drug compounds to expand our product candidate pipeline beyond CaPre. In such a scenario, we may not be able to identify and develop or acquire product candidates that prove to be successful products, or to develop or acquire them on terms that are acceptable to us.
CaPre could face competition from products for which no prescription is required.
If it receives regulatory approval, CaPre will be a prescription-only OM3. Mixtures of OM3 fatty acids are naturally occurring substances in various foods, including fatty fish. OM3 fatty acids are also marketed by other companies as dietary supplements or natural health products. Dietary supplements may generally be marketed without a lengthy FDA premarket review and approval process and do not require a prescription. However, unlike prescription drug products, manufacturers of dietary supplements may not make therapeutic claims for their products; dietary supplements may be marketed with claims describing how the product affects the structure or function of the body without premarket approval, but may not expressly or implicitly represent that the dietary supplement will diagnose, cure, mitigate, treat, or prevent disease. We cannot be certain that physicians or consumers will view CaPre as superior to these alternatives or that physicians will be more likely to prescribe CaPre. If the price of CaPre is significantly higher than the prices of commercially available OM3 fatty acids marketed by other companies as dietary supplements or natural health products, physicians may recommend these commercial alternatives instead of CaPre or patients may elect on their own to take commercially available non-prescription OM3 fatty acids. Either of these outcomes could limit how we price CaPre and negatively affect our revenues.
Even if we obtain FDA approval of CaPre, we may never obtain approval or commercialize it outside of the United States, which would limit our ability to realize CaPres full market potential.
In order to market CaPre outside of the United States, we must establish and comply with numerous and varying regulatory requirements of other countries regarding safety and efficacy. Clinical trials conducted in one country may not be accepted by regulatory authorities in other countries, and regulatory approval in one country does not
- 16 -
Table of Contents
mean that regulatory approval will be obtained in any other country. Approval procedures vary among countries and can involve additional clinical testing and validation and additional administrative review periods. Seeking foreign regulatory approvals could result in significant delays, difficulties and costs for us and may require additional preclinical studies or clinical trials, which would be costly and time consuming. Regulatory requirements can vary widely from country to country and could delay or prevent the introduction of CaPre in those countries. In addition, our failure to obtain regulatory approval in any country may delay or have negative effects on the process for regulatory approval in other countries. If we fail to comply with regulatory requirements in international markets or to obtain and maintain required approvals, our target market will be reduced and our ability to realize the full market potential of CaPre will be harmed.
If we or our third-party service providers fail to comply with healthcare laws and regulations or government price reporting laws, we could be subject to civil or criminal penalties.
In addition to the FDAs restrictions on marketing pharmaceutical products, several other types of federal and state healthcare fraud and abuse laws restrict marketing practices in the pharmaceutical industry. These laws include the U.S. Anti-Kickback Statute, U.S. False Claims Act and similar state laws. The U.S. Anti-Kickback Statute prohibits, among other things, offering, paying, soliciting or receiving remuneration to induce, or in return for, purchasing, leasing, or ordering any healthcare item or service reimbursable under Medicare, Medicaid or other federally financed healthcare programs. A person or entity does not need to have actual knowledge of the U.S. Anti-Kickback Statute or special intent to violate the law in order to have committed a violation. This statute has been interpreted broadly to apply to arrangements between pharmaceutical manufacturers and prescribers, dispensers, purchasers and formulary managers. The exemptions and safe harbors from prosecution are drawn narrowly and we may fail to meet all of the criteria for safe harbor protection from anti-kickback liability.
In addition, the Health Care Reform Law provides that the government may assert that a claim including items or services resulting from a violation of the U.S. Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the U.S. False Claims Act. Federal false claims laws prohibit any person from knowingly presenting, or causing to be presented, a false claim for payment to the federal government or knowingly making, or causing to be made, a false statement to get a false claim paid. The qui tam provisions of the False Claims Act allow a private individual to bring civil actions on behalf of the federal government alleging that the defendant has submitted a false claim to the federal government. These individuals, sometimes known as relators or, more commonly, as whistleblowers, may share in any amounts paid by the entity to the government in fines or settlement. The number of filings of qui tam actions has increased significantly in recent years, causing more healthcare companies to have to defend a case brought under the federal False Claim Act. If an entity is determined to have violated the federal False Claims Act, it may be required to pay up to three times the actual damages sustained by the government, plus attorneys fees and costs, and civil penalties of up to US$21,563 for each separate false claim. Certain administrative sanctions, up to and including exclusion of an entity from participation in the federal healthcare programs, may also ensue.
Additional laws and regulations include:
| | the U.S. federal Health Insurance Portability and Accountability Act (HIPAA), as amended by the Health Information Technology for Economic and Clinical Health Act (HITECH), which created additional federal criminal statutes that prohibit, among other things, schemes to defraud healthcare programs and imposes requirements on certain types of people and entities relating to the privacy, security, and transmission of individually identifiable health information, and requires notification to affected individuals and regulatory authorities of breaches of security of individually identifiable health information; |
| | the federal Physician Payment Sunshine Act, which requires certain manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid, or the Childrens Health Insurance Program, to report annually to the CMS information related to payments and other transfers of value to physicians, other healthcare providers and teaching hospitals, and ownership and investment interests held by physicians and other healthcare providers and their immediate family members, which is published in a searchable form on an annual basis; and |
- 17 -
Table of Contents
| | the U.S. Foreign Corrupt Practices Act and similar worldwide anti-bribery laws, which generally prohibit companies and their intermediaries from making improper payments to government officials for the purpose of obtaining or retaining business. Violations of these laws, or allegations of such violations, could result in fines, penalties or prosecution and have a negative impact on our business, results of operations and reputation. |
Over the past few years, a number of pharmaceutical and other healthcare companies have been prosecuted under these laws for a variety of alleged prohibited promotional and marketing activities, such as providing free trips, free goods, sham consulting fees and grants and other monetary benefits to prescribers; reporting to pricing services inflated average wholesale prices that were then used by federal programs to set reimbursement rates; engaging in off-label promotion that caused claims to be submitted to Medicaid for non-covered, off-label uses; and submitting inflated best price information to the Medicaid Rebate Program to reduce liability for Medicaid rebates. Most states also have statutes or regulations similar to the U.S. Anti-Kickback Statute and the U.S. False Claims Act, which apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor. Sanctions under these federal and state laws may include civil monetary penalties, exclusion of a manufacturers products from reimbursement under government programs, criminal fines and imprisonment. Settlements of U.S. government litigation may include Corporate Integrity Agreements with commitments for monitoring, training, and reporting designed to prevent future violations.
Any action against us for an alleged or suspected violation of these laws could cause us to incur significant legal expenses and could divert our managements attention from the operation of our business, even if our defense is successful. In addition, achieving and sustaining compliance with these laws and regulations may be costly to us in terms of money, time and resources. If we or any strategic partners, manufacturers or service providers fail to comply with these laws, we could be subject to enforcement actions, including:
| | adverse regulatory inspection findings; |
| | warning letters; |
| | voluntary or mandatory product recalls or public notification or medical product safety alerts to healthcare professionals; |
| | restrictions on, or prohibitions against, marketing our products; |
| | restrictions on, or prohibitions against, importation or exportation of our products; |
| | suspension of review or refusal to approve pending applications or supplements to approved applications; |
| | exclusion from participation in government-funded healthcare programs; |
| | exclusion from eligibility for the award of government contracts for our products; |
| | suspension or withdrawal of product approvals; |
| | product seizures; |
| | injunctions; and |
| | civil and criminal penalties and fines. |
We will rely on third parties to conduct our Phase 3 program for CaPre.
We will rely on contract research organizations, or CROs, to monitor and manage data for our Phase 3 program for CaPre. While we will only control certain aspects of the CROs activities, we nevertheless are responsible for ensuring that our clinical trials are conducted in accordance with applicable protocols, legal, regulatory and scientific standards, and our reliance on the CRO does not relieve us from those responsibilities. We and the CRO are required to comply with current good clinical practices, or cGCPs, which are regulations and guidelines enforced by the FDA, Health Canada and comparable foreign regulatory authorities for any products in clinical development.
- 18 -
Table of Contents
The FDA enforces these cGCP regulations through periodic inspections of trial sponsors, principal investigators and trial sites. If we or the CRO fail to comply with applicable cGCPs, the clinical data generated in our clinical trials may be deemed unreliable and the FDA, Health Canada or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving our marketing applications for CaPre. Upon inspection, the FDA could determine that our clinical trials do not comply with cGCPs. In addition, our clinical trials must be conducted with products produced under current good manufacturing practice, or cGMP, regulations and require a large number of test subjects. If we or the CRO fail to comply with these regulations, we may have to repeat preclinical studies or clinical trials for CaPre, which would delay the regulatory approval process and could also subject us to enforcement action up to and including civil and criminal penalties.
If our relationship with a CRO terminates, we may not be able to enter into arrangements with alternative CROs. If the CRO does not successfully carry out its duties or obligations or meet expected deadlines, if it needs to be replaced or if the quality or accuracy of the clinical data it obtains is compromised due to the failure to adhere to our clinical protocols, regulatory requirements or for other reasons, we may have to extend, delay or terminate our preclinical studies or clinical trials, and we may not be able to obtain regulatory approval for or successfully commercialize CaPre.
The third parties that will help conduct our Phase 3 Program for CaPre will not be our employees and, except for remedies available to us under our agreements with the CROs, we cannot control whether or not they devote sufficient time and resources to our preclinical, clinical and nonclinical programs. These third parties may also have relationships with other commercial entities, including our competitors, for whom they may also be conducting clinical studies or other drug development activities, which could affect their performance on our behalf.
We rely on third parties to manufacture, produce and supply CaPre and we may be adversely affected if those third parties are unable or unwilling to fulfill their obligations, including complying with FDA requirements.
Producing pharmaceutical products requires significant expertise and capital investment, including the development of advanced manufacturing techniques and process controls. Currently, we do not own or operate manufacturing facilities for the production of CaPre. Accordingly, we need to rely on one or more third party contract manufacturers to produce and supply our required drug product for our nonclinical research and clinical trials for CaPre.
Although we are currently working with CordenPharma at its Chenôve facility in Dijon, France to develop a commercially viable manufacturing process for CaPre, doing so is a difficult and uncertain task, and there are risks associated with scaling to the level required for commercialization, including, among others, cost overruns, potential problems with process scale up, process reproducibility, stability issues, lot consistency and timely availability of reagents or raw materials. We may not be able to attain our targeted cost of goods sold for CaPre. Any of these challenges could delay completion of our clinical trials for CaPre, require bridging or repetition of studies or trials, increase development costs, delay approval of CaPre, impair our commercialization efforts, and increase our costs. We may have to delay or suspend the production of CaPre if a third-party manufacturer:
| | becomes unavailable for any reason, including as a result of the failure to comply with cGMP regulations; |
| | experiences manufacturing problems or other operational failures, such as equipment failures or unplanned facility shutdowns required to comply with cGMP or damage from any event, including fire, flood, earthquake, business restructuring or insolvency; or |
| | fails or refuses to perform its contractual obligations under its agreement with us, such as failing or refusing to deliver the quantities of CaPre requested by us on a timely basis. |
If our third-party contract manufacturers fail to achieve and maintain high manufacturing standards in compliance with cGMP regulations, we may be subject to sanctions, including fines, product recalls or seizures, injunctions, delays or suspensions of our clinical trials for CaPre, total or partial suspension of production of
- 19 -
Table of Contents
CaPre, civil penalties, withdrawals of previously granted regulatory approvals, and criminal prosecution. We do not currently have arrangements in place for redundant supply. If any one of our current contract manufacturers cannot perform as agreed, we may be required to replace that manufacturer. Although we believe that there are several potential alternative contract manufacturers who could manufacture CaPre, we may incur added costs and delays in identifying and qualifying any such replacement.
Manufacture of CaPre involves using potentially hazardous materials.
Manufacturing activities relating to CaPre involve the controlled use of potentially hazardous substances, including chemical and biological materials, such as acetone. Our manufacturers for CaPre will be subject to federal, provincial, state and local laws and regulations in Canada, the United States and in other jurisdictions governing laboratory procedures and the use, manufacture, storage, handling and disposal of medical and hazardous materials. Although we believe that our manufacturers procedures for using, handling, storing and disposing of these materials comply with legally prescribed standards, we cannot completely eliminate the risk of contamination or injury resulting from medical or hazardous materials. If any such contamination or injury were to occur, we may incur liability or local, city, provincial, state or federal authorities may curtail the use of these materials and interrupt our business operations and the production of CaPre. In the event of an accident, we could be held liable for damages or penalized with fines, and the liability could exceed our resources. We do not have any insurance for liabilities arising from medical or hazardous materials. Complying with environmental, health and safety laws and regulations is expensive, and current or future environmental regulations may impair our research, development and production efforts relating to CaPre, which could harm our business, prospects, financial condition or results of operations. Although we maintain workers compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of hazardous materials, this insurance may not provide adequate coverage against potential liabilities. We do not maintain insurance for environmental liability or toxic tort claims that may be asserted against us in connection with our storage or disposal of biological, hazardous or radioactive materials. In addition, we may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. These laws and regulations may make it more difficult for us to conduct our research, development or production activities relating to CaPre and if we fail to comply with them, we could have substantial fines, penalties or other sanctions imposed against us.
We depend on Neptune for certain administrative and accounting services.
Neptune has provided us in the past with certain shared back office services and functions, including corporate affairs, public company reporting, accounting, payroll, information technology, accounts payable, accounts receivable and shared premises. As of the date of this prospectus, the corporate affairs and public company reporting services have not been renewed, so we are now incurring incremental costs, partially offset by reduced shared service fees, and expect to do so in the future, for providing these services independently or through qualified third parties. If our arrangements with Neptune for the remaining services were to be terminated or not renewed, we may have to incur additional costs to provide them ourselves or to source them from another third party.
In the past, Neptune supplied us with the krill oil needed to produce CaPre for our clinical programs, including the krill oil projected to be needed for our Phase 3 program, and we are now evaluating alternative supplies.
We have sourced all of our krill oil from Neptune in the past to produce CaPre. We have sufficient krill oil inventories that we anticipate will be required to complete our Phase 3 program. However, in light of Neptunes recent announcement of its plan to discontinue krill oil production and the sale of its krill oil inventory to Aker, we are evaluating alternative suppliers of krill oil. While we believe that alternative supplies of krill oil that can meet our specifications will be readily available, any alternative supply of krill oil may not be of comparable quality to that provided by Neptune, which could negatively affect the efficacy, or the markets perception of the efficacy, of CaPre. Our reliance on third-party suppliers for krill oil exposes us to risks such as potential fluctuations in supply and reduced control over our production costs and delivery schedules for CaPre.
- 20 -
Table of Contents
Interruptions of our supply of CaPre could impair any future revenue streams, if CaPre reaches commercialization.
We will require much larger amounts of CaPre than we have in the past if CaPre reaches commercialization. Supply interruptions for CaPre could occur and our inventory of CaPre may not always be sufficient due to a number of factors, including:
| | failure to have a third-party supply chain partners process validated in a timely manner; |
| | shortages of required raw materials, such as krill oil, and the packaging components required by our manufacturers; |
| | changes in our sources for manufacturing or packaging; |
| | failure to timely locate and obtain replacement manufacturers, as needed; and |
| | conditions affecting the cost and availability of raw materials. |
We are also in the process of scaling-up our production of CaPre, and CaPre may not be of comparable quality when produced in larger 200 kilogram or more batches. If we experience interruptions in the production of CaPre, our ability to complete our Phase 3 program could be interrupted. If CaPre receives regulatory approval, interruptions in the production of CaPre or insufficient inventory levels of CaPre could have a material adverse effect on our results of operations.
If product liability lawsuits are brought against us, we may incur substantial liabilities and be required to cease the sale, marketing and distribution of CaPre.
We face a potential risk of product liability associated with any future commercialization of CaPre or any other future product candidate we develop. For example, we may be sued if CaPre allegedly causes injury. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product, negligence, strict liability and a breach of warranties. Claims could also be asserted under U.S. state or Canadian provincial or other foreign consumer protection legislation. If we cannot successfully defend against product liability claims, we may incur substantial liabilities or be required to cease the sale, marketing and distribution of CaPre. Even successful defense against product liability claims would require significant financial and management resources. Regardless of the merits or eventual outcome, liability claims may result in:
| | decreased demand for CaPre or any future products that we may develop; |
| | injury to our reputation; |
| | costs to defend the related litigation; |
| | a diversion of managements time and our resources; |
| | substantial monetary awards to consumers, trial participants or patients; |
| | product recalls, withdrawals or labeling, marketing or promotional restrictions; |
| | loss of revenue; |
| | an inability to commercialize CaPre; and |
| | a decline in the price of our common shares. |
If we are unable to obtain and retain sufficient product liability insurance at an acceptable cost to protect against potential product liability claims, the commercialization of CaPre or any other product candidates we develop could be hindered or prevented. We currently carry product liability insurance, shared with Neptune, in the amount of $10.0 million in the aggregate. Any claim that may be brought against us could result in a court
- 21 -
Table of Contents
judgment or settlement in an amount that is not covered, in whole or in part, by our insurance or that is in excess of the limits of our insurance coverage. Our insurance policies also have various exclusions, and we may be subject to a product liability claim for which we have no coverage. In the event of a successful product liability claim against us, we may have to pay from our own resources any amounts awarded by a court or negotiated in a settlement that exceed coverage limitations or that is not covered by our insurance, and we may not have, or be able to obtain, sufficient funds to pay such amounts.
We may be subject to foreign exchange rate fluctuations.
Our reporting currency is the Canadian dollar. However, many of our expenses, such as CaPres chief manufacturing organizations production activities and certain CRO arrangements that we anticipate entering into for our Phase 3 program, currently are and/or are expected to be, denominated in foreign currencies, including European euros and U.S. dollars. Though we plan to implement measures designed to reduce our foreign exchange rate exposure, the U.S. dollar/Canadian dollar and European euro/Canadian dollar exchange rates have fluctuated significantly in the recent past and may continue to do so, which could have a material adverse effect on our business, financial position and results of operations.
Risks Related to Intellectual Property
It is difficult and costly to protect our intellectual property rights.
The success of our business will largely depend on our ability to:
| | obtain and maintain patents, trade secret protection and operate without infringing the intellectual proprietary rights of third parties; |
| | successfully defend our patents, including patents licensed to us by Neptune, against third-party challenges; and |
| | successfully enforce our patents against third party competitors. |
Our patents and/or proprietary technologies could be circumvented through the adoption of competitive, though non-infringing, processes or products. The patent positions of pharmaceutical companies can be highly uncertain and involve complex legal, scientific and factual questions for which important legal principles remain unresolved. Changes in either the patent laws or in interpretations of patent laws may diminish the value of our intellectual property. We cannot predict the breadth of claims that may be allowable or enforceable in our patents, including the patents licensed to us by Neptune.
We face risks that:
| | we may not be the first inventor of inventions covered by our issued patents or pending applications or be the first to file patent applications for those inventions; |
| | our pending or future patent applications may not be issued with the breadth of claim coverage sought by us, or be issued at all; |
| | our competitors could independently develop or patent technologies that are substantially equivalent or superior to our technologies; |
| | our trade secrets could be learned independently by our competitors; |
| | the steps we take to protect our intellectual property may not be adequate; |
| | effective patent, trademark, copyright and trade secret protection may be unavailable, limited or not sought by us in some foreign countries; and |
- 22 -
Table of Contents
| | our rights under our Canadian, U.S. or foreign patents or other patents that Neptune or other third parties license to us could be challenged. |
Further, patents have a limited lifespan. In the United States, a patent generally expires 20 years after it is filed (or 20 years after the filing date of the first non-provisional U.S. patent application to which it claims priority). While extensions may be available, the life of a patent, and the protection it affords, is limited. Without patent protection for CaPre or any other of our future product candidates, we may be open to competition from generic versions of CaPre or our other future product candidates. Further, the extensive period of time between patent filing and regulatory approval for a product candidate limits the time during which we can market that product candidate under patent protection. Patents owned by third parties could have priority over patent applications filed or in-licensed by us, or we or our licensors could become involved in interference, opposition or invalidity proceedings before U.S., Canadian or foreign patent offices. The cost of defending and enforcing our patent rights against infringement charges by other patent holders may be significant and could limit our operations.
In addition to our own patents, CaPre is covered by patents that are sublicensed to us by Neptune.
In addition to our proprietary patent applications, we have an exclusive worldwide license under a license agreement with Neptune to use certain patents and know-how to develop and commercialize CaPre within a specified field of use, namely the development and commercialization of CaPre and our novel and active pharmaceutical ingredients, or APIs, for the prescription drug and medical food markets. These patents were recently acquired by Aker BioMarine Antarctic AS, or Aker, from Neptune. Aker then licensed this intellectual property back to Neptune. Our license with Neptune remains in place and unchanged. This limitation on our field of use may prevent us from developing and commercializing CaPre in other fields.
Disputes may arise between us and Neptune or Aker regarding the intellectual property that is subject to the license agreement, including with respect to:
| | the scope of rights granted under the license agreement and other interpretation-related issues; and |
| | our right to sublicense patent and other rights to third parties under collaborative development relationships. |
If our sublicense with Neptune is terminated due to a breach by us of its terms (or should Neptunes license agreement with Aker otherwise terminate and we are unable to enter into a direct license agreement with Aker), we would not be able to manufacture and market CaPre, which would have a material adverse effect on our business and financial condition.
CaPre may infringe the intellectual property rights of others, which could increase our costs and delay or prevent our development and commercialization efforts.
Our success depends in part on avoiding infringement of the proprietary technologies of others. The pharmaceutical industry has been characterized by frequent litigation regarding patent and other intellectual property rights. Identification of third party patent rights that may be relevant to our proprietary or licensed technology is difficult because patent searching is imperfect due to differences in terminology among patents, incomplete databases and the difficulty in assessing the meaning of patent claims. Additionally, because patent applications are maintained in secrecy until the application is published, we may be unaware of third-party patents that may be infringed by our development and commercialization of CaPre or any other future product candidate. There may be certain issued patents and patent applications claiming subject matter that we may be required to license in order to research, develop or commercialize CaPre, and any such patents and patent applications may not be available to license on commercially reasonable terms, or at all. If claims of patent infringement are asserted by third parties against us, they could be time-consuming and may:
| | result in costly litigation; |
- 23 -
Table of Contents
| | divert the time and attention of our technical personnel and management; |
| | delay our clinical trials for CaPre; |
| | prevent us from commercializing CaPre until the asserted patent expires or is held finally invalid or not infringed in court; |
| | require us to cease or to modify our use of the technology and/or develop non-infringing technology; or |
| | require us to enter into royalty or licensing agreements. |
Others may hold proprietary rights that could prevent CaPre from being marketed. Any patent-related legal action against us claiming damages and seeking to enjoin commercial activities relating to CaPre or our processes could subject us to potential liability for damages and require us to obtain a license to continue to manufacture or market CaPre or any other future prescription drug candidates. We might not prevail in any such actions or if any license is required under any of these patents it may not be available on commercially acceptable terms, if at all.
Even if a license can be obtained on acceptable terms, the rights may be non-exclusive, which could give our competitors access to the same technology or intellectual property rights licensed to us. We could be forced to redesign CaPre or any other future product candidates or processes to avoid infringement.
In addition, we may find it necessary to pursue claims or initiate lawsuits to protect or enforce our patent or other intellectual property rights. The cost to us in defending or initiating any litigation or other proceeding relating to patent or other proprietary rights, even if resolved in our favor, could be substantial, and litigation would divert our managements attention. Some of our competitors may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could delay our research and development efforts and limit our ability to continue our operations.
A number of companies, including several major pharmaceutical companies, have conducted research on pharmaceutical uses of OM3 fatty acids, which has resulted in the filing of many patent applications related to this research. We are aware of third-party U.S., Canadian or other foreign patents that contain broad claims related to methods of using these general types of compounds, which may be construed to include potential uses of CaPre. If we were to challenge the validity of these or any other issued U.S., Canadian or other foreign patents in court, we would need to overcome a statutory presumption of validity that attaches to every U.S. and Canadian patent. This means that, in order to prevail, we would have to present clear and convincing evidence as to the invalidity of the other partys patents claims. If we were to challenge the validity of any issued U.S. patent in an administrative trial before the Patent Trial and Appeal Board in the United States Patent and Trademark Office, or USPTO, we would have to prove that the claims are unpatentable by a preponderance of the evidence. If there are disputes over our intellectual property rights, a jury and/or court may not find in our favor on questions of infringement, validity or enforceability.
If we do not protect our trademark for CaPre, we may not be able to build name recognition in our markets of interest.
We have trademarked CaPre. Our trademark may be challenged, infringed, circumvented or declared generic or determined to be infringing on other marks. We may not be able to protect our rights to this trademark or may be forced to stop using this name, which we need for name recognition by potential strategic partners and customers. If we are unable to establish name recognition based on our trademark, we may not be able to compete effectively and our business may be adversely affected.
- 24 -
Table of Contents
We may be involved in lawsuits to protect or enforce our patents or the patents of our licensors, which could be expensive, time- consuming and unsuccessful.
Competitors may infringe our patents or the patents of our licensors. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time-consuming. If we or our licensors were to initiate legal proceedings against a third party to enforce a patent covering CaPre or our technology, the defendant could counterclaim that our or our licensors patent is invalid or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements; for example, lack of novelty, obviousness or non-enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the USPTO, or made a misleading statement, during prosecution. The outcome following legal assertions of invalidity and unenforceability during patent litigation is unpredictable. With respect to the validity question, for example, we cannot be certain that there is no invalidating prior art, of which we or our licensors and the patent examiner were unaware during prosecution. If a defendant were to prevail on a legal assertion of invalidity or unenforceability, we would lose at least part, and perhaps all, of the patent protection on CaPre or certain aspects of our platform technology. Such a loss of patent protection could have a material adverse impact on our business. Patents and other intellectual property rights also will not protect our technology if competitors design around our protected technology without legally infringing our patents or other intellectual property rights.
In addition, in an infringement proceeding, a court may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. An adverse result in any litigation or defense proceedings could put one or more of our patents at risk of being invalidated, held unenforceable, or interpreted narrowly and could put our patent applications at risk of not issuing. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of management and employee resources from our business.
Interference proceedings provoked by third parties or brought by the USPTO may be necessary to determine the priority of inventions with respect to our patents or patent applications or those of our licensors. An unfavorable outcome could result in a loss of our current patent rights and could require us to cease using the related technology or to attempt to license rights to it from the prevailing party. Our business could be harmed if the prevailing party does not offer us a license on commercially reasonable terms, or at all. Litigation or interference proceedings may result in a decision adverse to our interests and, even if we are successful, may result in substantial costs and distract our management and other employees. We may not be able to prevent, alone or with our licensors, misappropriation of our trade secrets or confidential information, particularly in countries where the laws may not protect those rights as fully as in the United States. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common shares.
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Changes in patent law could diminish the value of patents in general, thereby impairing our ability to protect CaPre and any of our other future product candidates.
Numerous recent changes to the patent laws and proposed changes to the rules of the USPTO may have a significant impact on our ability to protect our technology and enforce our intellectual property rights. For
- 25 -
Table of Contents
example, the Leahy-Smith America Invents Act, or AIA, enacted in 2011, involves significant changes in patent legislation. An important change introduced by the AIA is that, as of March 16, 2013, the United States transitioned to a first-to-file system for deciding which party should be granted a patent when two or more patent applications are filed by different parties claiming the same invention. A third party that files a patent application in the USPTO after that date but before us could therefore be awarded a patent covering an invention of ours even if we had made the invention before it was made by the third party. This will require us to be cognizant going forward of the time from invention to filing of a patent application. Further, the Supreme Court of the United States has ruled on several patent cases in recent years, some of which cases either narrow the scope of patent protection available in certain circumstances or weaken the rights of patent owners in certain situations. These changes have led to increasing uncertainty with regard to the scope and value of our issued patents and to our ability to obtain patents in the future.
Among some of the other changes introduced by the AIA are changes that limit where a patentee may file a patent infringement suit and providing opportunities for third parties to challenge any issued patent in the USPTO. This applies to all of our U.S. patents, even those issued before March 16, 2013. Because of a lower evidentiary standard in USPTO proceedings compared to the evidentiary standard in United States federal court necessary to invalidate a patent claim, a third party could potentially provide evidence in a USPTO proceeding sufficient for the USPTO to hold a claim invalid even though the same evidence would be insufficient to invalidate the claim if first presented in a district court action. Accordingly, a third party may attempt to use the USPTO procedures to invalidate our patent claims that would not have been invalidated if first challenged by the third party as a defendant in a district court action.
Once granted, patents may remain open to opposition, interference, re-examination, post-grant review, inter partes review, nullification derivation and opposition proceedings in court or before patent offices or similar proceedings for a given period after allowance or grant, during which time third parties can raise objections against the initial grant. In the course of any such proceedings, which may continue for a protracted period of time, the patent owner may be compelled to limit the scope of the allowed or granted claims attacked, or may lose the allowed or granted claims altogether. Depending on decisions by the U.S. Congress, the U.S. federal courts, the USPTO or similar authorities in foreign jurisdictions, the laws and regulations governing patents could change in unpredictable ways that may weaken our and our licensors ability to obtain new patents or to enforce existing patents we and our licensors or partners may obtain in the future.
We may not be able to protect our intellectual property rights throughout the world.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of some countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets and other intellectual property protection, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
Risks Relating to Our Common Shares, Warrants and this Offering
There is a significant risk that we may be classified as a PFIC for U.S. federal income tax purposes.
Potential investors in our common shares and warrants who are U.S. holders should be aware that, based on our most recent financial statements and projections and given uncertainty regarding the composition of our future
- 26 -
Table of Contents
income and assets, there is a significant risk that we may be classified as a passive foreign investment company or PFIC for our taxable year ending March 31, 2018 and possibly subsequent years. If we are a PFIC for any year during a U.S. holders holding period of the common shares or warrants acquired pursuant to this prospectus, then such U.S. taxpayer generally will be required to treat any gain realized upon a disposition of such common shares or warrants or any so-called excess distribution received on such common shares or warrants, as ordinary income (with a portion subject to tax at the highest rate in effect), and to pay an interest charge on a portion of such gain or excess distribution. In certain circumstances, the sum of the tax and the interest charge may exceed the total amount of proceeds realized on the disposition, or the amount of excess distribution received, by the U.S. taxpayer. Subject to certain limitations, a timely and effective QEF Election under Section 1295 of the Code or a Mark-to-Market election under Section 1296 of the Code may be made with respect to the common shares. Neither the QEF Election nor the Mark-to-Market Election is available with respect to the warrants. A U.S. holder who makes a timely and effective QEF Election generally must report on a current basis its share of our net capital gain and ordinary earnings for any year in which we are a PFIC, whether or not we distribute any amounts to our shareholders. A U.S. holder who makes the Mark-to-Market Election generally must include as ordinary income each year the excess of the fair market value of their common shares over the holders basis therein. This paragraph is qualified in its entirety by the discussion below under the heading Certain U.S. Federal Income Tax ConsiderationsU.S. Federal Income Tax Considerations of the Acquisition, Ownership, and Disposition of Common SharesPassive Foreign Investment Company Rules. Each potential investor who is a U.S. holder should consult its own tax advisor regarding the U.S. federal, U.S. state and local, and non-U.S. tax consequences of the acquisition, ownership, and disposition of common shares and warrants acquired pursuant to this prospectus, the U.S. federal tax consequences of the PFIC rules, and the availability of any election that may be available to the holder to mitigate adverse U.S. federal income tax consequences of holding shares in a PFIC.
The trading price of our common shares may be volatile.
Market prices for securities in general, and those of pharmaceutical companies in particular, tend to fluctuate. The trading price for our common shares has experienced volatility in the past. Factors that could affect the trading price of our common shares and cause volatility include, among others:
| | results or delays of pre-clinical and clinical studies by us or others; |
| | the commencement, enrollment or results of future clinical trials we may conduct, or changes in the development status of CaPre or any of our other future product candidates; |
| | any delay in our regulatory filings for CaPre or any of our other future product candidates and any adverse development or perceived adverse development with respect to the applicable regulatory authoritys review of our filings; |
| | filing or granting or invalidity of patents; |
| | exclusive rights obtained by us or others; |
| | disputes or other developments relating to proprietary rights, including patents; |
| | litigation matters and our ability to obtain patent protection for our technologies; |
| | changes in regulations; |
| | additions or departures of key scientific or management personnel; |
| | overall performance of the equity markets; |
| | any failure to secure adequate capital to fund our operations on terms that are favorable to us, or at all; |
| | general political and economic conditions; |
| | publications; |
| | failure by us to meet the estimates and projections of the investment community or that we may otherwise provide to the public; |
- 27 -
Table of Contents
| | research reports or positive or negative recommendations or withdrawal of research coverage by securities analysts; |
| | announcements of significant acquisitions, strategic partnerships, joint ventures or capital commitments by us or our competitors; |
| | public concerns over the risks of pharmaceutical products and dietary supplements; |
| | unanticipated serious safety concerns related to the use of CaPre; and |
| | future sales of securities by us in financings or by our shareholders. |
As a result, the market price of our common shares may fluctuate significantly in the future. In addition, the stock market in general, and pharmaceutical companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. Broad market and industry factors may negatively affect the market price of our common shares, regardless of our actual operating performance. In the past, securities class action litigation has often been instituted against companies following periods of volatility of the market price of a companys securities. This type of litigation, if brought against us, could result in substantial costs and liabilities for us and divert our managements attention and resources, which would harm our business, operating results or financial condition.
Future securities issuances by us could result in significant dilution for existing shareholders.
Our articles of incorporation permit us to issue an unlimited number of common shares and preferred shares, issuable in series, and our shareholders will have no pre-emptive rights in connection with further issuances of securities by us. Our directors have the discretion to determine the provisions attaching to any series of preferred shares and the price of issue of further issuances of our common shares. Also, additional common shares may be issued by us upon the exercise of outstanding stock options and warrants or the conversion of debentures. The issuance of these additional equity securities or the issuance of new stock options or warrants may have a dilutive effect on existing holders of our common shares and, as a result, the market price for our common shares could decline. The market price of our common shares could also decline as a result of future issuances by us in connection with strategic partnerships, or sales by our existing shareholders, or the perception that these sales could occur. Sales by our shareholders, including Neptune, might also make it more difficult for us to sell equity securities at a time and price that we deem appropriate, which could reduce our ability to raise capital and have an adverse effect on our business.
Raising additional capital may adversely affect the rights of our existing shareholders, restrict our operations or require us to relinquish rights to our technologies or product candidates.
We may seek additional capital through a combination of public and private equity offerings, debt financings, strategic partnerships and alliances and licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interests of our shareholders will be diluted, and the terms may include liquidation or other preferences that adversely affect the rights of our common shareholders. The incurrence of indebtedness would result in increased fixed payment obligations and could involve certain restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. If we raise additional funds through strategic partnerships and licensing arrangements with third parties, we may have to relinquish valuable rights relating to CaPre or our other future product candidates, or grant licenses on terms unfavorable to us.
An active market for our common shares might not be sustained.
If an active market for our common shares is not sustained, holders of our common shares may be unable to sell their investments on satisfactory terms. Declines in the value of our common shares may adversely affect the
- 28 -
Table of Contents
liquidity of the market for our common shares. Factors unrelated to our performance may also have an effect on the price and liquidity of our common shares including:
| | extent of analyst coverage of us; |
| | lower trading volume and general market interest in our common shares; |
| | the size of our public float; and |
| | any event resulting in a delisting of our common shares from the NASDAQ Stock Market or the TSX Venture Exchange, or TSXV. |
A large number of our common shares may be issued and subsequently sold upon the exercise of our outstanding warrants and under our convertible debentures, which could depress the trading price for our common shares.
As of September 30, 2017, we had up to 5,254,535 common shares issuable under our outstanding warrants and convertible debentures. To the extent that holders of our warrants and convertible debentures sell underlying common shares issued under those warrants and convertible debentures, the market price of our common shares may decrease due to the additional selling pressure in the market and could encourage short sales by third parties. In a short sale, a prospective seller borrows common shares from a shareholder or broker and sells the borrowed common shares. The prospective seller anticipates that the common share price will decline, at which time the seller can purchase common shares at a lower price for delivery back to the lender. The risk of dilution from issuances of our common shares underlying our warrants and convertible debentures could also cause shareholders to sell their common shares, which could result in a decline in their market price.
We do not intend to pay dividends on our common shares for the foreseeable future.
We have never paid dividends on our common shares and we do not anticipate paying any dividends on our common shares for the foreseeable future because, among other reasons, we currently intend to retain any future earnings to finance our business. Any future payment of dividends by us will depend on factors such as cash on hand and whether we achieve profitability, our financial requirements to fund our growth, our general financial condition and other factors our board of directors may consider appropriate in the circumstances. Until we pay dividends, which we may never do, our shareholders will not be able to receive a return on their common shares unless they sell them.
There is no public market for the warrants being offered by us in this offering and an active trading market for the warrants is not expected to develop.
There is no established public trading market for the warrants being offered in this offering, and we do not expect a market to develop. In addition, we do not intend to apply for any listing of the warrants on the NASDAQ Stock Market, TSX Venture Exchange or any other securities exchange or nationally recognized trading system. Without an active market, the liquidity of the warrants will be limited.
The warrants are speculative in nature and may not have any value.
The warrants do not confer any rights of common share ownership on their holders, such as voting rights or the right to receive dividends, but rather merely represent the right to acquire common shares at a fixed price for a limited period of time. Specifically, commencing on the date of issuance, holders of the warrants may exercise their right to acquire common shares and pay an exercise price of US$1.26 per share, subject to certain adjustments, prior to the fifth anniversary of the date such warrants were issued, after which date any unexercised warrants will expire and have no further value. Moreover, following this offering, the market value of the warrants, if any, is uncertain and there can be no assurance that the market value of the warrants will equal or exceed their imputed offering price. The warrants will not be listed or quoted for trading on any market or
- 29 -
Table of Contents
exchange. There can be no assurance that the market price of the common shares will ever equal or exceed the exercise price of the warrants, and consequently, whether it will ever be profitable for holders of the warrants to exercise the warrants.
If we fail to meet applicable listing requirements, the NASDAQ Stock Market or the TSXV may delist our common shares from trading, in which case the liquidity and market price of our common shares could decline.
Our common stock is currently listed on the NASDAQ Stock Market and the TSXV, but we cannot assure you that our securities will continue to be listed on the NASDAQ Stock Market and the TSXV in the future. In the past, we have received notices from the NASDAQ Stock Market that we have not been in compliance with its continued listing standards, and we have taken responsive actions and regained compliance. If we fail to comply with listing standards and the NASDAQ Stock Market or TSXV delists our common shares, we and our shareholders could face significant material adverse consequences, including:
| | a limited availability of market quotations for our common shares; |
| | reduced liquidity for our common shares; |
| | a determination that our common shares are penny stock, which would require brokers trading in our common shares to adhere to more stringent rules and possibly result in a reduced level of trading activity in the secondary trading market for our common shares; |
| | a limited amount of news about us and analyst coverage of us; and |
| | a decreased ability for us to issue additional equity securities or obtain additional equity or debt financing in the future. |
We may pursue opportunities or transactions that adversely affect our business and financial condition.
In the ordinary course of our business, our management regularly explores potential strategic opportunities and transactions, which may involve:
| | significant debt or equity investments in us by third parties; |
| | the acquisition or disposition by us of material assets; |
| | the licensing, acquisition or disposition by us of material intellectual property; |
| | the development of new product lines or new applications for our existing products; |
| | entering into distribution arrangements; |
| | issuance of our common shares; and |
| | other similar matters. |
Public announcement by us of strategic opportunities or transactions might have a significant effect on the trading price of our common shares. Our policy is to not publicly disclose our pursuit of a potential strategic opportunity or transaction unless we are required to do so by applicable law. Investors who buy or sell our common shares could be doing so at a time when we are pursuing a particular strategic opportunity or transaction that, when announced, could have a significant effect on the trading price for our common shares.
In addition, any strategic transactions we enter into could carry significant risks, including:
| | exposure to unknown liabilities; |
| | higher than anticipated transaction costs and expenses; |
| | the difficulty and expense of integrating operations and personnel of any acquired companies; |
- 30 -
Table of Contents
| | disruption of our ongoing business; |
| | diversion of our managements time and attention; and |
| | possible dilution to our existing shareholders. |
As a foreign private issuer, we are subject to different U.S. securities laws and regulations than a domestic U.S. issuer, which may limit the information publicly available to our U.S. shareholders.
We are a foreign private issuer under applicable U.S. federal securities laws, and therefore, we are not required to comply with all the periodic disclosure and current reporting requirements of the U.S. Securities and Exchange Act of 1934, or the Exchange Act. As a result, we do not file the same reports that a U.S. domestic issuer would file with the SEC, although we are required to file with or furnish to the SEC the continuous disclosure documents that we are required to file in Canada under Canadian securities laws. In addition, our officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions of Section 16 of the Exchange Act. Therefore, our shareholders may not know on as timely a basis when our officers, directors and principal shareholders purchase or sell common shares as the reporting periods under the corresponding Canadian insider reporting requirements are longer. In addition, as a foreign private issuer, we are exempt from the proxy rules under the Exchange Act.
As an emerging growth company, we are exempt from the requirement to comply with the auditor attestation requirements of the Sarbanes-Oxley Act.
We are an emerging growth company, as defined in the U.S. Jumpstart Our Business Start-ups Act, and we use the exemption provided to emerging growth companies from the auditor attestation requirements of Section 404(b) of the Sarbanes-Oxley Act of 2002. Therefore, our internal controls over financial reporting will not receive the level of review provided by the process relating to the auditor attestation included in annual reports of issuers that are not using an exemption. In addition, we cannot predict if investors will find our common shares less attractive because we rely on this exemption. If some investors find our common shares less attractive as a result, there may be a less active trading market for our common shares and trading price for our common shares may be negatively affected.
U.S. investors may be unable to enforce certain judgments against us because of our Canadian incorporation and presence.
We are a company existing under the Business Corporations Act (Québec). Some of our directors and officers are residents of Canada, and substantially all of our assets are located outside the United States. As a result, it may be difficult to effect service within the United States upon us or upon some of our directors and officers. Execution by U.S. courts of any judgment obtained against us or any of our directors or officers in U.S. courts may be limited to assets located in the United States. It may also be difficult for holders of securities who reside in the United States to realize in the United States upon judgments of U.S. courts predicated upon civil liability of us and our directors and executive officers under the U.S. federal securities laws. There may be doubt as to the enforceability in Canada against non-U.S. entities or their controlling persons, directors and officers who are not residents of the United States, in original actions or in actions for enforcement of judgments of U.S. courts, of liabilities predicated solely upon U.S. federal or state securities laws.
We have broad discretion in how we use the net proceeds of the offering, and we may not use these proceeds in a manner desired by our shareholders.
We will have broad discretion with respect to the use of the net proceeds from the offering and investors will be relying on the judgment of our management regarding the application of these proceeds. We could spend most of the net proceeds from the offering in ways that our shareholders may not desire or that do not yield a favorable return. You will not have the opportunity, as part of your investment in our securities, to influence the manner in which the net proceeds of the offering are used. We currently intend to use the proceeds of the offering as described in Use of Proceeds. However, our needs may change as our business and our industry evolve. As a result, the proceeds we receive in the offering may be used in a manner significantly different from our current expectations.
- 31 -
Table of Contents
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains information that may be forward-looking statements within the meaning of applicable securities laws. Forward-looking statements can be identified by the use of terms such as may, will, should, expect, plan, anticipate, believe, intend, estimate, predict, potential, continue or other similar expressions concerning matters that are not statements about the present or historical facts. Forward-looking statements in this prospectus include, among other things, or statements about:
| | our ability to conduct all required clinical and nonclinical trials for CaPre, including the timing and results of those clinical trials; |
| | our strategy, future operations, prospects and the plans of our management; |
| | the design, regulatory plan, timeline, costs and results of our clinical and nonclinical trials for CaPre; |
| | the timing and outcome of our meetings and discussions with the U.S. Food and Drug Administration, or FDA; |
| | our planned regulatory filings for CaPre, and their timing; |
| | our expectation that our Bridging Study (as defined below) results will support our plan to get authorization from the FDA to use the its 505(b)(2) pathway with new chemical entity, or NCE, status towards a New Drug Application, or NDA, approval in the United States; |
| | the timing and results from two competitor outcomes studies in patients with high TGs (blood levels between 200-499 mg/dL); |
| | the potential benefits and risks of CaPre as compared to other products in the pharmaceutical, medical food and natural health products markets; |
| | our anticipated marketing advantages and product differentiation of CaPre and its potential to become a best-in-class omega-3, or OM3, compound for the treatment of severe HTG (very high blood levels of TGs over 500 mg/dL); |
| | our estimates of the size of the potential market for CaPre, unmet medical needs in that market, the potential for market expansion, and the rate and degree of market acceptance of CaPre if it reaches commercialization, and our ability to serve that market; |
| | the potential to expand CaPres indication for the treatment of high TGs; |
| | the degree to which physicians would switch their patients to a product with CaPres target product profile; |
| | our strategy and ability to develop, commercialize and distribute CaPre in the United States and elsewhere; |
| | the manufacturing scale-up of CaPre and the related timing; |
| | our intention and ability to strengthen our patent portfolio and other means of protecting our intellectual property rights; |
| | the availability, consistency and sources of our raw materials, including krill oil; |
| | our expectation to be able to rely on third parties to manufacture CaPre whose manufacturing processes and facilities are in compliance with current good manufacturing practices, or cGMP; |
| | the potential for OM3s in other cardiovascular medicine, or CVM, indications; |
| | our intention to pursue development and/or distribution partnerships to support the development and commercialization of CaPre, and to pursue strategic opportunities to provide capital and market access; |
| | our ability to reach a definitive agreement based upon our non-binding term sheet with a leading China-based pharmaceutical company for the commercialization of CaPre in certain Asian countries; |
- 32 -
Table of Contents
| | our need for additional financing and our estimates regarding our future financing and capital requirements; |
| | our expectation regarding our financial performance, including our revenues, profitability, research and development, costs and expenses, gross margins, liquidity, capital resources, capital expenditures and our access to additional capital; and |
| | our projected capital requirements to fund our anticipated expenses, including our research and development and general and administrative expenses. |
All forward-looking statements reflect our belief and assumptions based on information available at the time the assumption was made. The forward-looking statements in this prospectus are subject to a number of known and unknown risks, uncertainties and other factors, including those described in this prospectus under Risk Factors, many of which are beyond our control, that could cause our actual results and developments to differ materially from those that are disclosed in or implied by the forward-looking information, including, among others:
| | risks related to timing and possible difficulties, delays or failures in our Phase 3 program for CaPre; |
| | pre-clinical and clinical trials may be more costly or take longer to complete than anticipated, and may never be initiated or completed, or may not generate results that warrant future development of CaPre; |
| | we may fail to achieve our publicly announced milestones on time; |
| | outcome study data from two of our competitors in high TGs patients may be negative, which could also negatively affect the market perception of CaPre; |
| | there may be difficulties, delays, or failures in obtaining health care reimbursements for CaPre; |
| | the market opportunity for, and demand and market acceptance of, CaPre may not be as strong as we anticipate; |
| | we have significant additional future capital needs and may not be able to raise additional financing required to fund further research and development, clinical studies, obtain regulatory approvals, and meet ongoing capital requirements to continue our current operations on commercially acceptable terms or at all; |
| | CaPre may not prove to be as safe and effective or as potent as we currently believe; |
| | our Phase 3 program may not produce positive results; |
| | our anticipated studies and submissions to the FDA may not occur as currently anticipated, or at all; |
| | the FDA could reject our 505(b)(2) regulatory pathway; |
| | we may encounter difficulties, delays or failures in obtaining regulatory approvals for the initiation of clinical trials or to market CaPre; |
| | we may need to conduct additional future clinical trials for CaPre, the occurrence and success of which cannot be assured; |
| | CaPre may have unknown side effects; |
| | the FDA may refuse to approve CaPre, or place restrictions on our ability to commercialize CaPre; |
| | CaPre could be subject to extensive post-market obligations and continued regulatory review, which may result in significant additional expense and affect sales, marketing and profitability; |
| | we may encounter difficulties in completing the development and commercialization of CaPre; |
| | third parties we will rely upon to conduct our Phase 3 program for CaPre may not effectively fulfill their obligations to us, including complying with FDA requirements; |
| | recently enacted and future laws may increase the difficulty and cost for us to obtain marketing approval of and commercialize CaPre and affect the prices we can charge; |
- 33 -
Table of Contents
| | new laws, regulatory requirements, and the continuing efforts of governmental and third-party payors to contain or reduce the costs of healthcare through various means could adversely affect our business; |
| | third parties that we will rely upon to manufacture, supply and distribute CaPre may not effectively fulfill their obligations to us, including complying with FDA requirements; |
| | there may not be an adequate supply of raw materials, including krill oil, in sufficient quantities and quality and to produce CaPre under cGMP standards; |
| | Neptune has significant influence with respect to matters submitted to our shareholders for approval; |
| | Neptunes interest may not align with those of us or our other shareholders; |
| | we may not be able to meet applicable regulatory standards for the manufacture of CaPre or scale-up our manufacturing successfully; |
| | we may not be able to produce future clinical batches, if needed, and commercial batches of CaPre in a timely manner or at all; |
| | we currently have no sales, marketing and distribution personnel; |
| | our patent applications may not result in issued patents, our issued patents may be circumvented or challenged and ultimately struck down, and we may not be able to successfully protect our trade secrets or other confidential proprietary information; |
| | we may face claims of infringement of third party intellectual property and other proprietary rights; |
| | we sublicense intellectual property that has been recently sold by Neptune to Aker (and then licensed back to Neptune). Although our license agreement with Neptune remains in place, our rights under the sublicense agreement are subject to the continued term of the license between Neptune and Aker; |
| | we may face product liability claims and product recalls; |
| | we face intense competition from other companies in the pharmaceutical, medical food and natural health product industries; |
| | we have a history of negative operating cash flow and may never become profitable or be able to sustain profitability; |
| | we may not be able to attain our targeted cost of goods sold, and levels of insurance reimbursement for CaPre may not be commercially viable in all global markets; |
| | we may acquire businesses or products or form strategic partnerships in the future that may not be successful; |
| | we may be unable to secure development and/or distribution partnerships to support the development and commercialization of CaPre, provide development capital, or market access; |
| | we recently entered into a non-binding term sheet with a leading China-based pharmaceutical company that would grant it an exclusive right to commercialize CaPre in certain Asian countries, and it is possible that no definitive agreement with the China-based company will be reached, or if a definitive agreement is reached its terms may differ from those in the term sheet; |
| | we rely on key management and skilled scientific personnel; and |
| | general changes in economic and capital market conditions could adversely affect us. |
All of the forward-looking statements in this prospectus are qualified by this cautionary statement. There can be no guarantee that the results or developments that we anticipate will be realized or, even if substantially realized, that they will have the consequences or effects on our business, financial condition or results of operations that we anticipate. As a result, you should not place undue reliance on the forward-looking statements. Except as required by applicable law, we do not undertake to update or amend any forward-looking statements, whether as a result of new information, future events or otherwise. All forward-looking statements are made as of the date of this prospectus.
- 34 -
Table of Contents
The following table presents the average exchange rate for one Canadian dollar expressed as one U.S. dollar for each of our last five fiscal years. The average rate is calculated using the average of the exchange rates on the last day of each month during the period.
| Fiscal year ended | Average | |||
| (US$) | ||||
| February 28, 2013 |
0.9903 | |||
| February 28,2014 |
0.9555 | |||
| February 28, 2015 |
0.8003 | |||
| February 29, 2016 |
0.7645 | |||
| March 31, 2017 |
0.7618 | |||
The following table presents the high and low exchange rate for one Canadian dollar expressed as one U.S. dollar for each month during the previous six months.
| Month | Low | High | ||||||
| (US$) | ||||||||
| June 2017 |
0.7405 | 0.7706 | ||||||
| July 2017 |
0.7703 | 0.8034 | ||||||
| August 2017 |
0.7840 | 0.8012 | ||||||
| September 2017 |
0.8013 | 0.8245 | ||||||
| October 2017 |
0.7756 | 0.8018 | ||||||
| November 2017 |
0.7759 | 0.7885 | ||||||
The exchange rates above are based upon the noon buying rate, as quoted by the Bank of Canada. As of May 1, 2017, the Bank of Canada no longer publishes updated data for exchange rates published under previous methodologies, including daily noon and closing rates as well as high and low exchange rates. For the month of May 2017 and each month thereafter, the exchange rate presented above is based upon the daily average closing rate. As of December 20, 2017, the exchange rate for one Canadian dollar expressed as one U.S. dollar, as quoted by the Bank of Canada was $1.00 = US$0.7787.
- 35 -
Table of Contents
We estimate that we will receive net proceeds of approximately US$8.6 million (or approximately US$9.6 million if the underwriters option to purchase additional common shares and warrants is exercised in full) from the sale of common shares and warrants offered by us in this offering and after deducting the estimated underwriting discounts and estimated offering expenses payable by us.
If all of the warrants sold in this offering came to be exercised in cash, we would receive additional net proceeds of approximately US$11.2 million. We cannot predict when or if these warrants will be exercised. It is possible that the warrants may expire and may never be exercised.
We currently intend to use the net proceeds of this offering, together with our cash on hand, for the further development of CaPre, including clinical site activation, progression of patient enrollment and production of clinical materials (both CaPre and placebo) for our Phase 3 program; expansion of business development activities; working capital; and other general corporate purposes.
Our expected use of net proceeds from this offering represents our current intentions based upon our present plans and business condition, which could change in the future as our plans and business conditions evolve. The amounts and timing of our actual use of the net proceeds will depend on multiple factors, including the progress, cost and results of our preclinical and clinical development programs, competitive and technological developments, strategic partnering activities, and the overall regulatory environment. As a result, our management will retain broad discretion in the application of the net proceeds, and investors will be relying on our judgment regarding the application of the net proceeds from this offering.
Pending our use of the net proceeds from this offering, we may plan to invest the net proceeds in short- and intermediate-term, interest-bearing obligations, investment-grade instruments, certificates of deposit or government securities, or hold them as cash.
- 36 -
Table of Contents
We do not anticipate paying any cash dividend on our common shares in the foreseeable future. We presently intend to retain future earnings to finance the development and growth of our business. Any future determination to pay dividends will be at the discretion of our board of directors and will depend on our financial condition, results of operations, capital requirements and other factors the board of directors deems relevant. In addition, the terms of any future debt or credit facility may preclude us from paying dividends. Any remittances of dividends to United States residents and to other non-residents are, however, subject to withholding tax. See Certain U.S. Federal Income Tax Considerations and Certain Canadian Federal Income Tax Considerations for additional information.
- 37 -
Table of Contents
The table below sets forth our total indebtedness and shows our capitalization as at September 30, 2017:
| | on an actual basis; and |
| | on an as adjusted basis, to give effect to the issuance and sale of 9,900,990 common shares at offering price of US$1.01 per share and warrants to purchase an aggregate of 8,910,891 common shares, after deducting underwriting discounts and estimated offering expenses. We assumed (i) no exercise of warrants sold in this offering, and (ii) that the exchange rate for Canadian dollars expressed in United States dollars of the offering was $1.00 = US$0.7787 (the daily average exchange rate for one Canadian dollar expressed in United States dollars as reported by the Bank of Canada on December 20, 2017). |
You should read this table together with our financial statements and accompanying notes included in this prospectus, and the other information appearing in Selected Financial Information and Managements Discussion and Analysis of Financial Condition and Results of Operations.
| As at September 30, 2017 | ||||||||
| (actual) | (as adjusted) | |||||||
| (in thousands) | ||||||||
| Cash and cash equivalents |
$ | 5,329 | $ | 16,389 | ||||
|
|
|
|
|
|||||
| Non-current liabilities |
||||||||
| Derivative warrant liabilities(1)(2) |
51 | 5,514 | ||||||
| Unsecured convertible debentures |
1,509 | 1,509 | ||||||
|
|
|
|
|
|||||
| Total non-current liabilities |
1,560 | 7,362 | ||||||
|
|
|
|
|
|||||
| Equity |
||||||||
| Share capital(1)(2) |
66,633 | 73,164 | ||||||
| Other equity |
309 | 309 | ||||||
| Contributed surplus |
6,024 | 6,024 | ||||||
| Deficit(2) |
(58,160 | ) | (59,094 | ) | ||||
|
|
|
|
|
|||||
| Total equity |
$ | 14,806 | 20,403 | |||||
|
|
|
|
|
|||||
| Total capitalization |
$ | 16,366 | 27,426 | |||||
|
|
|
|
|
|||||
| (1) | The warrants are a derivative liability (Derivative Warrant Liabilities) for accounting purposes due to the currency of the exercise price (US$) being different from our Canadian dollar functional currency. The proceeds of this offering are required to be split between the Derivative Warrant Liabilities and the equity-classified common shares at the time of issuance. Because the warrants are not listed on a national securities exchange or other nationally recognized trading market, the underwriters will be unable to satisfy any overallotment of shares and warrants without exercising the underwriters overallotment option with respect to the warrants. As a result, the underwriters will exercise their overallotment option for all of the warrants which are over-allotted, if any, at the time of the initial offering of the shares and the warrants. However, because our common shares are publicly traded, the underwriters may satisfy some or all of the overallotment of common shares, if any, by purchasing shares in the open market and will have no obligation to exercise the overallotment option with respect to our common stock. For purposes of the as adjusted amounts in the table and splitting the proceeds of this offering between the Derivative Warrant Liabilities and the equity-classified common shares at the time of issuance, the fair value of the Derivative Warrant Liabilities excludes the 990,099 overallotment warrants issuable for nominal proceeds to the Company, despite the fact that the underwriters will exercise their overallotment option for all of the warrants which are over-allotted. The fair value of the Derivative Warrant Liabilities has been calculated using an assumed common stock price of US$1.01. The allocation of proceeds is a preliminary estimate and the actual allocation may be different by a material amount. |
- 38 -
Table of Contents
| (2) | After deducting the underwriters discounts of US$700,000, our estimated offering expenses of US$688,000 and the estimated fair value of the underwriter warrants of US$321,000 (determined using a Black-Scholes valuation model with an assumed common share price of US$1.01). For purposes of the as adjusted amounts in the table, the underwriter discount, our estimated offering expenses and the underwriter warrants have been allocated on a relative value basis to the common shares for an amount of US$982,000 and to the Derivative Warrant Liabilities for an amount of US$727,000. The portion allocated to the common shares is netted against its book value and the portion allocated to the Derivative Warrant Liabilities will be recorded in earnings, reflected in deficit in the as adjusted amounts in the table. |
The number of common shares outstanding as of September 30, 2017 on an adjusted basis is 24,636,927 common shares. The number of our common shares to be outstanding after this offering is based on 14,735,937 of our common shares outstanding as of September 30, 2017. The number of common shares to be outstanding after this offering excludes the following:
| | 2,402,188 common shares issuable upon the exercise of options issued to our directors, officers and employees, at a weighted-average exercise price of $1.82 per common share; |
| | 1,052,630 common shares issuable upon conversion of debentures at an exercise price of $1.90 per common share; |
| | 1,840,000 common shares issuable upon the exercise of warrants at a weighted-average exercise price of US$15.00 per common share; |
| | 161,654 common shares issuable upon the exercise of warrants at a weighted-average exercise price of $13.30 per common share; |
| | 1,965,259 common shares issuable upon the exercise of warrants at an exercise price of $2.15 per common share; |
| | 234,992 common shares issuable upon the exercise of broker warrants at an exercise price of $2.15 per common share; |
| | 495,050 common shares issuable upon the exercise of underwriter warrants at an exercise price of US$1.26 per common share issued in connection with this offering; and |
| | 8,910,891 common shares issuable upon the exercise of warrants to be issued to investors in connection with this offering at an exercise price of US$1.26 per common share. |
- 39 -
Table of Contents
If you invest in our securities in this offering, your ownership interest will be immediately diluted to the extent of the difference between the public offering price per share and the pro forma net tangible book value per share after this offering.
Our historical net tangible book value as of September 30, 2017 was approximately $3.6 million, or $0.25 per common share. Our historical net tangible book value is the amount of our total tangible assets less our liabilities. Net historical tangible book value per share is our historical net tangible book value divided by the number of common shares outstanding as of September 30, 2017.
Our pro forma net tangible book value as of September 30, 2017 was $14.7 million, or $0.60 per common share. Pro forma net tangible book value is our pro forma net tangible book value, plus the effect of the sale of common shares and warrants in this offering at a public offering price of US$1.01 per share and accompanying warrant, and after deducting underwriting discounts and estimated offering expenses payable by us. This amount represents an immediate increase in pro forma net tangible book value of approximately $0.35 per share to our existing shareholders, and an immediate dilution of approximately $0.70 per share to new investors participating in this offering.
The following table illustrates this dilution on a per share basis:
| Public offering price per share |
$ | 1.30 | ||||||
| Historical net tangible book value per share as of September 30, 2017 |
$ | 0.25 | ||||||
| Increase (decrease) in net tangible book value per share attributable to new investors participating in this offering |
0.35 | |||||||
| Pro forma net tangible book value per share as of September 30, 2017, after giving effect to this offering |
0.60 | |||||||
|
|
|
|||||||
| Dilution per share to new investors purchasing our common shares in this offering |
$ | 0.70 |
If the underwriters exercise their option to purchase additional common shares and warrants in full, the pro forma net tangible book value will increase to $0.62 per share, representing an immediate dilution of $0.68 per share to new investors purchasing our securities in this offering. For purposes of the foregoing discussion, the public offering price of US$1.01 per share and accompanying warrant has been converted into Canadian dollars based on the daily average exchange rate for one U.S. dollar expressed as one Canadian dollar, as quoted by the Bank of Canada of US$1.00 = $0.7787, as of December 20, 2017.
The discussion of dilution, and the table quantifying it, assumes no exercise of any outstanding options or warrants, including warrants issued under this offering, or other potentially dilutive securities. The exercise of potentially dilutive securities having an exercise price less than the offering price would increase the dilutive effect to new investors.
If the proceeds of the offering were reduced by the Derivative Warrant Liabilities of $5.5 million and excluding the effects of the underwriters option to purchase additional common shares and warrants and excluding all dilutive instruments, the pro forma net tangible book value will decrease to $0.37 per share, representing an immediate dilution of $0.93 per share to new investors purchase. The pro forma net tangible book value was computed including the full proceeds of the offering (i.e., without including the proceeds for the Derivative Warrant Liabilities).
- 40 -
Table of Contents
The number of our common shares to be outstanding after this offering is based on 14,735,937 of our common shares outstanding as of September 30, 2017, and excludes the following:
| | 2,402,188 common shares issuable upon the exercise of options issued to our directors, officers and employees, at a weighted-average exercise price of $1.82 per common share; |
| | 1,052,630 common shares issuable upon conversion of debentures at an exercise price of $1.90 per common share; |
| | 1,840,000 common shares issuable upon the exercise of warrants at a weighted-average exercise price of US$15.00 per common share; |
| | 161,654 common shares issuable upon the exercise of warrants at a weighted-average exercise price of $13.30 per common share; |
| | 1,965,259 common shares issuable upon the exercise of warrants at an exercise price of $2.15 per common share; |
| | 234,992 common shares issuable upon the exercise of broker warrants at an exercise price of $2.15 per common share; |
| | 495,050 common shares issuable upon the exercise of underwriter warrants at an exercise price of US$1.26 per common share issued in connection with this offering; and |
| | 8,910,891 common shares issuable upon the exercise of warrants to be issued to investors in connection with this offering at an exercise price of US$1.26 per common share. |
- 41 -
Table of Contents
Overview
We are a biopharmaceutical innovator focused on the research, development and commercialization of prescription drugs using omega-3, or OM3, fatty acids derived from krill oil. OM3 fatty acids have extensive clinical evidence of safety and efficacy in lowering triglycerides, or TGs, in patients with hypertriglyceridemia, or HTG. Our lead product candidate is CaPre, an OM3 phospholipid, which we are developing initially for the treatment of severe HTG, a condition characterized by very high levels of TGs in the bloodstream (³ 500 mg/dL). Market research commissioned by us from DP Analytics in 2016 suggests there is a significant unmet medical need for an effective, safe and well-absorbing OM3 therapeutic that demonstrates a positive impact on the major blood lipids associated with cardiovascular disease risk. We believe that, if supported by our Phase 3 program in the United States, which we initiated during the second half of 2017 and for which we plan to start clinical site activation by the end of 2017, CaPre will address this unmet medical need. We also believe the potential exists to expand CaPres initial indication to the high TGs (200 499 mg/dL) segment, although at least one additional clinical trial would likely be required to expand CaPres indications to this segment. We may seek to identify new potential indications for CaPre that may be appropriate for future studies and pipeline expansion. In addition, we may also seek to in-license other cardiometabolic drug candidates for drug development and commercialization.
In four clinical trials conducted to date, we saw the following beneficial effects with CaPre, and we are seeking to demonstrate similar results in our Phase 3 program:
| | significant reduction of TGs and non-high density lipoprotein cholesterol (non-HDL-C) levels in the blood of patients with mild to severe HTG; |
| | no deleterious effect on low-density lipoprotein cholesterol (LDL-C), or bad cholesterol, with the potential to reduce LDL-C; |
| | potential to increase high-density lipoprotein cholesterol (HDL-C), or good cholesterol; |
| | good bioavailability (absorption by the body), even under fasting conditions; |
| | no significant food effect when taken with either low-fat or high-fat meals; and |
| | an overall safety profile similar to that demonstrated by currently marketed OM3s. |
Our Successful Phase 1 and Phase 2 Studies Help Reduce Phase 3 Program Risk
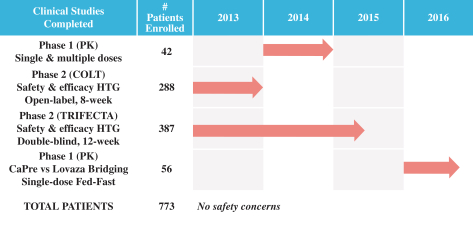
- 42 -
Table of Contents
About Hypertriglyceridemia
According to the American Heart Association Scientific Statement on Triglycerides and Cardiovascular Disease from 2011, TG levels provide important information as a marker associated with the risk for heart disease and stroke, especially when an individual also has low levels of HDL-C and elevated levels of LDL-C. HTG can be caused by both genetic and environmental factors, including obesity, sedentary lifestyle and high-calorie diets. HTG is also associated with comorbid conditions such as chronic renal failure, pancreatitis, nephrotic syndrome and diabetes. Multiple epidemiological, clinical, genetic studies suggest that patients with elevated TG levels (³ 200 mg/dL) are at a greater risk of coronary artery disease, or CAD, and pancreatitis, a life-threatening condition, as compared to those with normal TG levels. The genes regulating TGs and LDL-C are equally strong predictors of CAD, but HDL-C is not. Other studies suggest that lowering and managing TG levels may reduce these risks. In addition, the Japan EPA Lipid Intervention Study, or JELIS, demonstrated the long-term benefit of an OM3 eicosapentaenoic acid, or EPA, in preventing major coronary events in hypercholesterolemic patients receiving statin treatment. JELIS found a 19% relative risk reduction in major coronary events in patients with relatively normal TGs but a more pronounced 53% reduction in the subgroup with TGs > 150mg/dL and HDL-C < 40mg/dL. Recently published meta-analyses by Alexander et al. (Mayo Clinic Proceedings, 2017) and Maki et al. (Journal of Clinical Lipidology, 2016) suggest that EPA and docosahexaenoic acid, or DHA, may be associated with reducing coronary heart disease risk to a greater extent in populations with elevated TG levels, and that drugs lowering TG and TG-rich lipoproteins may reduce cardiovascular event risk in patients with elevated TG levels, particularly if associated with low HDL-C.
About CaPre
CaPre is a krill oil-derived mixture containing polyunsaturated fatty acids, or PUFAs, primarily composed of OM3 fatty acids, principally EPA and DHA. EPA and DHA are well known to be beneficial for human health, and according to numerous recent clinical studies, may promote healthy heart, brain and visual function, and may also contribute to reducing inflammation and blood TGs. Krill is a natural source of phospholipids and OM3 fatty acids. The EPA and DHA contained in CaPre are delivered as a combination of OM3s as free fatty acids and OM3s bound to phospholipid esters, allowing these PUFAs to reach the small intestine where they undergo rapid absorption and transformation into complex fat molecules that are required for lipid transport in the bloodstream. We believe that EPA and DHA are more efficiently transported by phospholipids sourced from krill oil than the EPA and DHA contained in fish oil that are transported either by TGs (as in dietary supplements) or as ethyl esters in other prescription OM3 drugs (such as LOVAZA and VASCEPA), which must then undergo additional digestion before they are ready for transport into the bloodstream. The digestion and absorption of OM3 ethyl ester drugs requires a particular enzymatic process that is highly dependent on the fat content of a mealthe higher the fat content, the better the OM3 ethyl ester absorption. High fat content meals are not recommended in patients with HTG. We believe that CaPres superior absorption profile could represent a significant clinical advantage, since taking it with a low-fat meal represents a more realistic regimen for patients with HTG who must follow a restricted low-fat diet.
CaPre is intended to be used as a therapy combined with positive lifestyle changes, such as a healthy diet, and to be administered either alone or with other drug treatment regimens such as statins (a class of drug used to reduce LDL-C). CaPre is intended to be taken orally once or twice per day in capsule form.
Potential Market for CaPre
We believe a significant opportunity exists for OM3 market expansion because, among other things:
| | cardiovascular diseases, or CVD, and stroke are the leading causes of morbidity and mortality in the United States. The burden of CVD and stroke in terms of life-years lost, diminished quality of life, and direct and indirect medical costs also remains enormous; |
| | evidence suggests potential for OM3s in other cardiometabolic indications; and |
- 43 -
Table of Contents
| | based on the assumption that the REDUCE-IT trial sponsored by Amarin and the STRENGTH trial sponsored by Astra Zeneca, or the CV outcome trials, will be positive, key opinion leaders interviewed by DP Analytics in the study described further below estimated that they would increase their own prescribing of OM3s by 42% in high TGs patients (200 499 mg/dL) and by 35% in severe HTG patients. For more information on the potential risks to our company from these trials, see Risk FactorsIf outcome studies being conducted by two of our competitors testing the impact of OM3 on treating patients with high TGs are negative, there could also be an adverse impact for CaPre. |
According to the American Heart Association, the prevalence of HTG in the United States and globally correlates to the aging of the population and the increasing incidence of obesity and diabetes. The American Heart Association has estimated that one-third of adults in the United States have elevated levels of TGs (TGs >150 mg/dL), including approximately 36 million people diagnosed with high TGs, and 3 to 4 million people diagnosed with severe HTG. Moreover, according to Ford et al. in a study conducted between 1999 and 2004, 18% of adults in the United States, corresponding to approximately 40 million people, had elevated TG levels equal to or greater than 200 mg/dl, of which only 3.6% were treated specifically with TG-lowering medication. We believe this data indicates there is a large underserved market opportunity for CaPre.
In 2015, CaPres target market in the United States for treatment of HTG was estimated by IMS NSP Audit data to be approximately US$750 million, with approximately 5 million prescriptions written annually over the prior four years. The total global market for treatment of HTG was estimated by GOED Proprietary Research in 2015 to be approximately US$2.3 billion. Currently, all marketed OM3 products are approved by the FDA only for patients with severe HTG. We believe there is the potential to greatly expand the treatable market in the United States to the approximately 36 million people with high TGs, assuming favorable results from the CV outcome studies that are currently ongoing. These CV outcome trials are expected to report in mid-2018 (the REDUCE-IT trial sponsored by Amarin) and 2019 (the STRENGTH trial sponsored by AstraZeneca) and are designed to evaluate the long-term benefit of lowering TGs on cardiovascular risks with prescription drugs containing OM3 fatty acids. If these trials are successful, additional clinical trials would likely be required for CaPre to also expand its label claims to the high TGs segment. Given the large portion of the adult population in the United States that have elevated levels of TGs but who go largely untreated, we believe there is the potential for a very significant increase in the total number of patients eligible for treatment if the CV outcome trials are positive.
The following charts illustrate the estimated global and U.S. markets for HTG in 2015, according to IMS NSP Audit data:
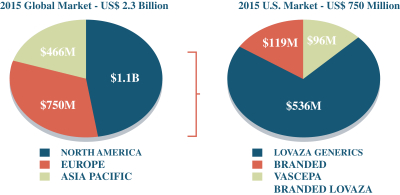
CaPre has two FDA-approved and marketed branded competitors (LOVAZA and VASCEPA). In addition, Astra Zeneca has an FDA-approved product, EPANOVA, which has not yet been launched. LOVAZA generics became available on the U.S. market in 2013. In spite of generic options, audited prescription data from IMS NSP Audit
- 44 -
Table of Contents
suggests that over 50% of OM3 prescriptions are written for branded products (LOVAZA or VASCEPA). By 2015, there had been only an approximately 25% decline in total market value, in spite of some generic switching that occurs at pharmacies. This stability of branded products is due in part to the fact that the pricing differential between branded and generic OM3 products is smaller than is often the case between branded and generic products in the pharmaceutical industry. Based on both primary market research with pharmacy benefit managers, or PBMs, and audited prescription reports, the average pricing of generics is currently approximately US$190 per month, while pricing for branded products averages US$250US$300 per month. Amarin has raised prices for VASCEPA annually since its launch in late 2013. PBMs offer Preferred Brand status (Tier 2 or Tier 3), without significant restrictions (i.e. no prior authorization, step edits, or high co-payments) for these branded OM3s.
Except as otherwise indicated, all of the information that follows under this heading has been derived from secondary sources, including audited U.S. prescribing data, and from a qualitative U.S. commercial and primary market research assessment conducted for us by DP Analytics, A Division of Destum Partners, Inc., or Destum, a market research firm, dated August 19, 2016, which we refer to as the Destum Market Research. In its market analysis for CaPre, Destum utilized secondary market data and reports and conducted primary qualitative market research with physicians and third-party payers, such as PBMs. One-on-one in-depth phone interviews lasting on average 30-60 minutes were conducted with 22 physicians and 5 PBMs, and key qualitative data was obtained by Destum on current clinical practice for treating patients with HTG, and their perceptions of the current unmet medical need in treating patients with HTG. All interviews were conducted by the same individual at Destum and recorded to ensure consistency and collection of key data points. Destum utilized OM3 prescription data from 2009 to 2015 to estimate the size of CaPres potential market. Based on its discussions with the PBMs, Destum also assumed CaPre would be viewed favorably by payers at launch (e.g., Tier 2 or 3, depending on payer plan, which is comparable to LOVAZA and VASCEPA). Upon completing the screening questionnaire and being approved for inclusion in Destums study, key opinion leaders, or KOLs, and high volume prescribers, or HVPs, were provided with a study questionnaire and were asked to comment on a target profile for a potential new OM3 Product X offering a trifecta of cardio-metabolic benefits similar to the potential efficacy and safety benefits demonstrated by CaPre in our two Phase 1 pharmacokinetic studies and two Phase 2 clinical trials, which we refer to as the Target Product Profile. Respondents were told that the unidentified product was being prepared for a Phase 3 program designed to confirm with statistical significance the products safety and efficacy in patients with severe HTG. The Target Product Profile was used by Destum strictly for market research analysis purposes and should not be construed as an indication of future performance of CaPre and should not be read as an expectation or guarantee of future performance or results of CaPre, and will not necessarily be an accurate indication of whether or not such results will be achieved by CaPre in our Phase 3 program. We subsequently retained Destum as our exclusive advisor and business development consultant to identify potential strategic partners for CaPre, under which Destum may be entitled to a success fee if a business arrangement or transaction is consummated. Destums market research and its conclusions were substantially completed prior our entry into this agreement with Destum.
During the Destum Market Research, KOLs and HVPs interviewed by Destum were asked to assess the level of unmet medical need associated with treating patients with HTG based on currently available treatment options. 91% of physicians interviewed by Destum indicated that they believe that the current unmet medical need for treating HTG was moderate to high. The reasons identified by these physicians for their dissatisfaction with the currently available OM3s included insufficient lowering of TGs (principally relating to VASCEPA), negative LDL-C effects (principally relating to LOVAZA), gastrointestinal side effects, and the fishy taste from fish oil-derived OM3s. Despite the availability of other drug classes to treat HTG, interviewed physicians indicated that they would welcome the introduction of new and improved OM3 products, particularly if they can address these perceived deficiencies.
Interviewed physicians responded favorably in the Destum Market Research to the Target Product Profile. They indicated that their weighted prescribing percentages of the Target Product Profile would increase by approximately 35% to 53% (with the range depending on the specific profile presented) of their HTG patients
- 45 -
Table of Contents
within two years of the Target Product Profiles approval. Approximately 60% of the interviewed physicians indicated that they would switch primarily due to the trifecta effect of the Target Product Profile on reducing TGs and LDL-C while elevating HDL-C, and the remaining 40% indicated they would switch primarily due to the Target Product Profiles effective reduction of TGs alone. In connection with their responses, the interviewed physicians were instructed to assume the Target Product Profile and all currently available OM3 products were not subject to any reimbursement or coverage hurdles (e.g., all products were on an equal health care coverage playing field). This assumption was supported by our interviews with leading PBMs in the United States.
We plan to conduct additional market research with KOLs, HVPs, primary care physicians and payers to further develop and refine our understanding of the potential marketplace for CaPre.
Our Clinical Data
CaPre is being developed by us initially for the treatment of patients with severe HTG. In two Phase 2 clinical trials conducted by us in Canada (our COLT and TRIFECTA trials), CaPre was found to be safe and well-tolerated at all doses tested, with no serious adverse events that were considered treatment-related. Among the reported adverse events with an occurrence of greater than 2% of subjects and greater than placebo, only diarrhea had an incidence of 2.3%.
In both Phase 2 clinical trials, CaPre significantly lowered TGs in patients with mild to severe HTG. Importantly, in these studies, CaPre also demonstrated no deleterious effect on LDL-C (unlike LOVAZA and EPANOVA, which have been shown to significantly increase LDL-C in patients with severe HTG). Further, our Phase 2 data indicated that CaPre may actually reduce LDL-C. LDL-C is undesirable because it accumulates in the walls of blood vessels, where it can cause blockages (atherosclerosis). In the Phase 2 trials, CaPre also reduced non-HDL-C (all cholesterol contained in the bloodstream except HDL-C), which is also considered to be a marker of cardiovascular disease. The COLT trial data showed a mean increase of 7.7% in HDL-C with CaPre at 4 grams per day (p=0.07). Further studies in our Phase 3 program are required to demonstrate CaPres statistical significance with HDL-C.
We believe that these potential multiple cardiovascular benefits, if confirmed in our Phase 3 program, could be significant differentiators for CaPre in the marketplace, as no currently approved OM3 drug has shown an ability to positively modulate these four major blood lipid categories (TGs, non-HDL-C, LDL-C and HDL-C) in the treatment of severe HTG. We also believe that if supported by additional clinical trials, CaPre has the potential to become a best-in-class OM3 compound for the treatment of patients with high TGs.
On September 14, 2016, we announced positive data from our completed comparative bioavailability study, or the Bridging Study. The Bridging Study was an open-label, randomized, four-way, cross-over, bioavailability study comparing CaPre, given as a single dose of 4 grams in fasting and fed (high-fat) states, as compared to the FDA-approved HTG drug LOVAZA (OM3-acid ethyl esters) in 56 healthy volunteers. The protocol was reviewed and approved by the FDA. The primary objective of the Bridging Study was to compare the bioavailability of CaPre to LOVAZA, each administered as a single 4 gram dose with a high-fat meal, which is the condition under which administration of OM3 drugs will yield the highest levels of EPA and DHA in the blood, and therefore has the highest potential for toxicity. To allow us to rely on the long-term safety data of LOVAZA to support a 505(b)(2) NDA for CaPre, our results had to show that the blood levels of EPA and DHA resulting from a single 4 gram dose of CaPre are not significantly higher than from a single 4 gram dose of LOVAZA under fed (high-fat meal) conditions. The Bridging Study met all of its objectives and demonstrated that the levels of EPA and DHA following administration of CaPre did not exceed corresponding levels following administration of LOVAZA in subjects who were fed a high-fat meal. We expect that these results will support a claim by us that CaPre and LOVAZA have a comparable safety profile. Also, among subjects in a fasting state, CaPre demonstrated better bioavailability than LOVAZA, as measured by significantly higher blood levels of EPA and DHA. Since most HTG patients must follow a restricted low-fat diet, we believe that CaPres strong bioavailability profile compared to OM3 ethyl ester drugs such as LOVAZA and VASCEPA could provide a more effective clinical solution for these patients.
- 46 -
Table of Contents
We summarized and submitted data from our Bridging Study to the FDA for review and discussed it with the FDA at an End-of-Phase 2 meeting during the first quarter of 2017. We also presented our Bridging Study data at the National Lipid Association Conference in May 2017 and we plan to submit the data from our Bridging Study for peer review and publication.
The graph below illustrates that the Bridging Study achieved all of its objectives:
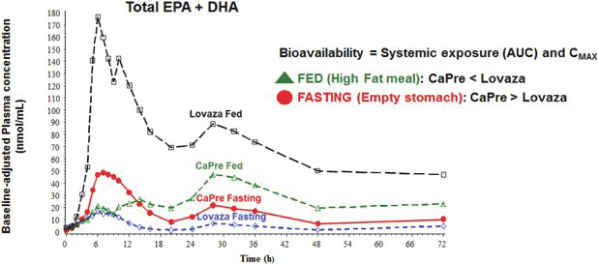
Absorption of EPA and DHA as ethyl ester formulations in the currently available prescription OM3 drugs derived from fish oil (such as LOVAZA and VASCEPA) require the breakdown of the ethyl esters by pancreatic enzymes (lipases) to be released into the blood. These particular enzymes are produced in response to the consumption of high-fat content meals, leading to optimal absorption of EPA and DHA. As a result, these OM3 ethyl ester formulations have demonstrated lower absorption and bioavailability when taken with a low-fat meal or on an empty stomach. As shown in our CAP13-101 study described further below, absorption of CaPre, which is formulated as OM3 phospholipids and free fatty acids, is not meaningfully affected by the fat content of a meal consumed prior to drug administration. Since a low-fat diet is typically a critical component for treatment of patients with severe HTG, we believe that being able to effectively combine CaPre with a low-fat diet could give CaPre a significant clinical and marketing advantage over the ethyl ester-based OM3s, such as LOVAZA and VASCEPA, that must be taken with a high-fat meal to achieve optimal absorption.
Our CAP13-101 study was an open-label, randomized, multiple-dose, single-center, parallel-design study in healthy volunteers. 42 subjects were enrolled into 3 groups of 14 subjects who took 1 gram, 2 grams or 4 grams of CaPre, administered once a day 30 minutes after breakfast. The objectives of the study were to determine the pharmacokinetic, or PK, profile and safety on Day 1 following a single oral dose and Day 14 following multiple oral doses of CaPre in individuals pursuing a low-fat diet (therapeutic lifestyle changes diet). The effect of a high-fat meal on the bioavailability of CaPre was also evaluated at Day 15. Blood samples were collected for assessment of EPA and DHA total lipids in plasma to derive the PK parameters.
The PK profile of CaPre following multiple 4 gram doses obtained in the CAP13-101 study at Day 14 was compared to the results obtained in a similar PK study (Offman 2013ECLIPSE 2) where LOVAZA was also administered at 4 grams a day for 14 days with a low-fat diet. Although CaPre contains approximately 2.5 times less EPA and DHA compared to LOVAZA (approximately 310 mg/1g capsule for CaPre versus 770 mg/1g capsule for LOVAZA), when administered with a low-fat meal, CaPre plasma levels of EPA and DHA are very similar to those of LOVAZA, as indicated by the area under the plasma drug concentration against time curve, or AUC, and the maximal plasma drug concentration. This study provided us with the basis for the dosing and design of our Phase 3 program.
- 47 -
Table of Contents
As illustrated by the two graphs below, CaPre reached similar blood and therapeutic levels to LOVAZA after 14 daily doses of CaPre at 4 grams/day, despite CaPre containing 2.5 times less EPA and DHA compared to LOVAZA:
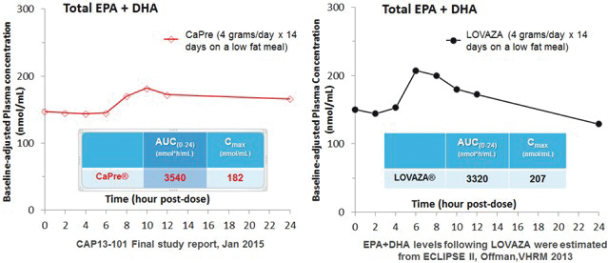
The graph below illustrates that the bioavailability of CaPre (total EPA+DHA levels in the blood) does not appear to be meaningfully affected by the fat content of a meal after multiple daily doses of CaPre at 4 grams/day (< 20% difference in AUC). We believe that CaPres strong bioavailability could represent a significant clinical advantage for CaPre since taking it with a low-fat meal represents a more realistic regimen for patients with HTG who must follow a restricted low-fat diet.
Our Study CAP13-101 CaPre Pharmacokinetics Shows No Significant Food Effect
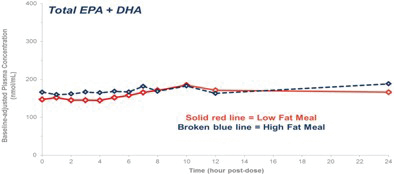
The graph below presents a summary of the effects of CaPre on patients lipid profiles as obtained in our completed TRIFECTA and COLT Phase 2 clinical trials. 90% of the patients in these clinical trials had high TGs (levels between 200 499 mg/dL) and 10% of patients had severe HTG (levels between 500 and 877 mg/dL), which was the maximum level of TGs permitted by Health Canadas study protocol. Only 30% of the participating patients were taking statins, which we believe is important because statins appear to enhance the TG-lowering effect of OM3s. In contrast, in our competitors summary data that follows, 100% of the patients in those studies with high TGs were taking statins with their OM3s.
The summary data from our COLT and TRIFECTA clinical trials shows that CaPre significantly reduces TGs, but unlike some other prescription EPA/DHA-based OM3s, it has no deleterious effect on LDL-C and may
- 48 -
Table of Contents
potentially increase HDL-C (p=0.07), which we refer to as the trifecta effect. Also, a dose response was seen for all of the major lipid markers; the greater the dose of CaPre, the greater the beneficial effect of CaPre.
Our Phase 2 Study Results Show CaPre Dose Response and Potential for Trifecta Lipid Effect
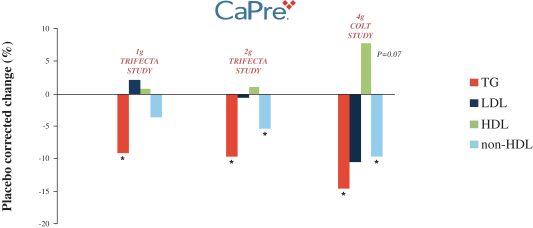
| * | Indicates results reached statistical significance |
TRIFECTA for 1g (N=130) & 2g (N=128) and COLT for 4g (N=62). HDL-C results at 4g from COLT approached statistical significance at P=0.07.
We conducted a subgroup analysis including only patients with severe HTG, consisting of approximately 10% of the patients from our TRIFECTA study, to compare the effects of CaPre versus other OM3 drugs in the initial target population of patients with severe HTG. Despite being given at a lower dose (only 1 gram and 2 grams), CaPres results compared very well with data from independent studies for the other prescription OM3 drugs that are FDA-approved for the treatment of severe HTG at higher doses of 2 grams and 4 grams. While the results of this subgroup analysis were not statistically significant for CaPre (which we believe is potentially due to the small sample size), numerically, the results compared well with the other OM3 drugs, even though CaPre was given at a much lower dose. The results for LDL-C, HDL-C and non-HDL-C levels in the subgroup shown in the table below are based on descriptive statistics only and are solely directional, meaning that no statistical testing was conducted and so no p values were generated.
- 49 -
Table of Contents
Our Sub-Group Analysis in Patients with Severe HTG: CaPre1 at 1g and 2g Compares Well with Our Competitors2 at 2g and 4g
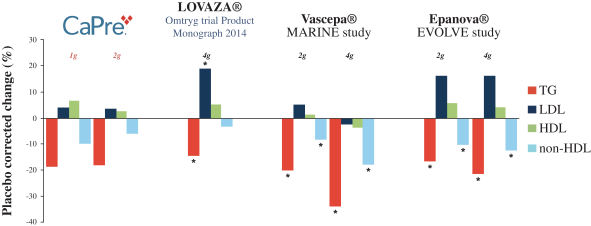
Only ~1/3 of all patients across all studies were on statins
| * | Indicates results reached statistical significance |
| 1. | Subgroup analysis on CaPre Phase 2 TRIFECTA study data in patients with severe HTG; (N=10 for 1g & N=14 for 2g). Results are not statistically significant for TGs, which may be explained by the small number of patients in this subgroup analysis. Results for LDL-C, HDL-C and non-HDL-C are based on descriptive statistics only (no statistical testing conducted). |
| 2. | LOVAZA 4g (N=103), VASCEPA 2g/4g (N=73/76), EPANOVA 2g/4g (N=100/99). |
Since statins appear to enhance the TG-lowering property of OM3 drugs, we conducted a subgroup analysis that only included patients who were taking a statin at baseline in the COLT and TRIFECTA studies (approximately 30% of the population of both trials, combined). The graph below compares the TG-lowering effects of CaPre to other OM3s, all on a background of a statin drug, and shows that CaPres TG-lowering effects compare well with other FDA-approved OM3 drugs. We believe it is noteworthy that only 39 patients on 2 grams of CaPre in our TRIFECTA study (out of a total of 128) and only 22 patients on 4 grams of CaPre in our COLT study (out of 62) were taking statins.
The CaPre 2 gram bar graph in the table below shows the results from patients in our TRIFECTA trial who were taking statins. A statistically significant reduction in TGs (-25.7% placebo corrected) was seen in that statin subgroup. The CaPre 4 gram bar graph in the table below shows patient results only from our COLT trial (as there was no 4 gram component for our TRIFECTA). None of the results were statistically significant at 4 grams of CaPre, which we believe is potentially due to the small number of patients (22) in the statins subgroup.
As seen in the larger full study analyses in the tables above, CaPre does not show any deleterious effect on LDL, and shows the potential to decrease LDL and increase HDL (p=0.07). These observations will need to be confirmed in our Phase 3 program.
- 50 -
Table of Contents
Our Sub-Group Analysis in Patients Treated with Statins1 vs Independent Competitor Data2: Potential for CaPre Trifecta Effect
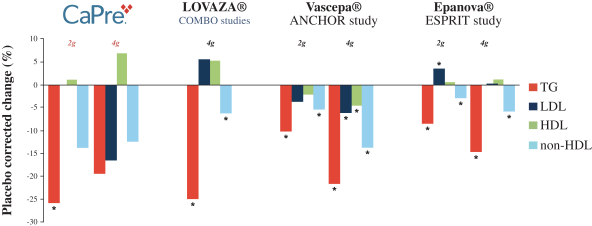
| * | Indicates results reached statistical significance |
| 1. | CaPre subgroup analyses on patients treated with statins: TRIFECTA for 2g (N=39) and COLT for 4g (N=22). For CaPre 2g, results for LDL-C, HDL-C, and non-HDL-C are based on descriptive statistics only (no statistical testing was conducted). For CaPre 4g, no results are statistically significant which may be explained by the small number of patients. |
| 2. | All patients on a statin background: LOVAZA (N=122 for 4g), VASCEPA (N= 234 for 2g, N=227 for 4g), EPANOVA (N=209 for 2g, N=207 for 4g). Statins have been shown to enhance the efficacy of OM3 productsVASCEPA NDA 202057. Statistical review, section 4.2 Other special/Subgroup populations, p. 107; and Maki K et al. Clin. Ther. 2013. |
In summary, in addition to effectively reduce TG levels in patients with mild to severe HTG, clinical data collected by us to date indicates that CaPre may also have:
| | beneficial effects on other blood lipids, such as HDL-C (good cholesterol) and non-HDL-C; |
| | no deleterious effect on, and may potentially reduce, LDL-C (bad cholesterol) levels; and |
| | absorption capability that is not meaningfully affected by the fat content of a meal consumed prior to drug administration, providing patients with the reassurance that following their physician-recommended low-fat diet will still result in high absorption. |
We believe that these features could set CaPre apart from currently available FDA-approved OM3 treatment options in the marketplace and could give us a significant clinical and marketing advantage.
- 51 -
Table of Contents
Based on our Phase 2 clinical trial results, CaPres potential clinical benefits as compared to currently available FDA-approved OM3 treatment options are summarized in the table below and indicate that CaPre may deliver a more complete lipid management solution for patients with severe HTG:
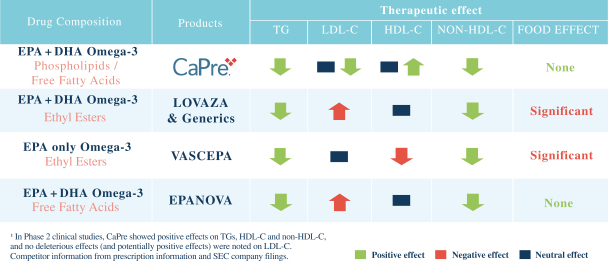
Our Nonclinical Research
In addition to our Phase 2 clinical trials, we carried out an extensive nonclinical program to demonstrate the safety of CaPre in a defined set of studies required by the FDA. These studies were carried out by contract research organizations with Good Laboratory Practice certification and conducted on various species of animals recommended by the FDA to investigate the long-term effects of CaPre at doses of up to 65 grams of human equivalent dose over 39 weeks. In these studies, hematological, biochemical, coagulation and overall health parameters of CaPre were evaluated and no toxic effects were observed in any of the segments of the studies. Other studies focused on the potential toxic effects of CaPre on vital systems, such as the cardiovascular, respiratory and central nervous system as evaluated by behavioral studies of the various species. These studies showed that CaPre did not have any adverse or toxic effects on any of the vital systems investigated, again up to doses well above the recommended clinical dose of CaPre. To rule out short term toxic effects of CaPre on genes, genomic toxicity studies were undertaken on accepted cellular and animal models. These studies showed no toxic effects of CaPre on any of the genetic markers indicative of potential gene altering toxic effects.
We believe the studies conducted to date indicate that CaPre is well-tolerated and shows no significant toxic effects on any of the physiological and vital systems of the tested animals or their genes or molecules at doses well above CaPres anticipated clinical therapeutic dose of 4 grams daily.
In parallel to our Phase 3 program, we will have to complete additional nonclinical studies, including a pre- and postnatal development study in rodents and a 26-week oral carcinogenicity study in transgenic hemizygous rasH2 mice. These nonclinical studies will be required to support an NDA for CaPre.
Our Phase 3 Program Design
In March 2017, we announced our plans to proceed with our Phase 3 program following our End-of-Phase 2 meeting with the FDA in February 2017. Based on the guidance we received from the FDA, we plan to conduct two pivotal, randomized, placebo-controlled double-blinded Phase 3 studies to evaluate the safety and efficacy of CaPre in patients with severe HTG. These studies of 26 weeks duration will evaluate CaPres ability to lower TGs from baseline in approximately 500 patients randomized to either 4 grams daily or placebo. The FDAs feedback supported our plan to conduct two studies in parallel, potentially shortening the time to an NDA
- 52 -
Table of Contents
submission. These studies will be conducted in multiple centers across North America. The primary endpoint of these studies is to determine the efficacy of CaPre at 4 grams/day compared to placebo in lowering TGs in severe HTG patients, and to confirm safety. In addition, the Phase 3 studies will include numerous secondary and exploratory endpoints, which are designed to assess the effect of CaPre on the broader lipid profile and certain metabolic, inflammatory and CV risk markers. If any of these secondary or exploratory endpoints show statistical significance, they could become the basis for possible expanded claims and/or future indications for CaPre.
We have initiated our Phase 3 program and expect to begin site activation before the end of 2017, subject to obtaining the required financing through this offering. We are working with a major clinical research organization to prepare for site activation and to manage our Phase 3 program, and we recently announced that Dariush Mozaffarin, M.D., Ph.D., has agreed to serve as our principal investigator. Dr. Mozaffarin is a cardiologist and epidemiologist serving as the Jean Mayer Professor of Nutrition & Medicine, and the Dean of the Friedman School of Nutrition Science & Policy at Tuffs University. His widely published research focuses on how diets, such as those rich in OM3s and lifestyle influence cardiometabolic health, and how effective policies can improve health and wellness.
The following chart illustrates the expected design and dosing of our Phase 3 program for CaPre.
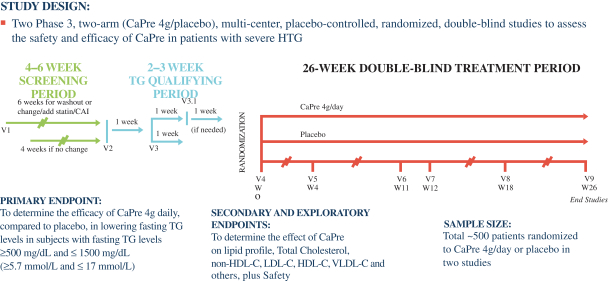
Our Regulatory Strategy for CaPre
Our strategy is to develop and initially commercialize CaPre for the treatment of severe HTG. Our Phase 3 program is designed to fully evaluate the clinical effect of CaPre on TGs, non-HDL-C, LDL-C, and HDL-C levels together with a variety of other cardiometabolic biomarkers in patients with severe HTG.
In December 2015, we announced that we intend to pursue a 505(b)(2) regulatory pathway towards an NDA approval in the United States. A 505(b)(2) regulatory pathway is defined in the U.S. Federal Food, Drug, and Cosmetic Act (FDCA) as an NDA containing investigations of safety and effectiveness that are being relied upon for approval and were not, in whole, conducted by or for the applicant, and for which the applicant has not obtained a right of reference. 505(b)(2) regulatory pathways differ from a typical NDA because they allow a sponsor to rely, at least in part, on the FDAs findings of safety and/or effectiveness for a previously-approved drug. We intend to pursue the 505(b)(2) regulatory pathway as a strategy to leverage the large body of safety data for LOVAZA, which could accelerate and streamline the development of CaPre and reduce associated costs and risks.
In connection with our intended use of the 505(b)(2) pathway, the FDA supported our proposal to conduct our Bridging Study that compared CaPre (which has an OM3 free fatty acid/phospholipid composition) with the
- 53 -
Table of Contents
FDA-approved HTG drug LOVAZA (which has an OM3-acid ethyl esters composition) in healthy volunteers. In February 2017, we met with the FDA to review our Bridging Study data. We confirmed with the FDA the 505(b)(2) regulatory approach to use the safety data for LOVAZA, and finalized the study design for our Phase 3 clinical trials, which will be required for NDA approval. We expect to continue our dialogue with the FDA during the second half of 2017 to obtain feedback on our regulatory and clinical plans and to clarify or answer specific questions regarding our Phase 3 clinical studies.
Our planned key milestones and development timeline are presented below.
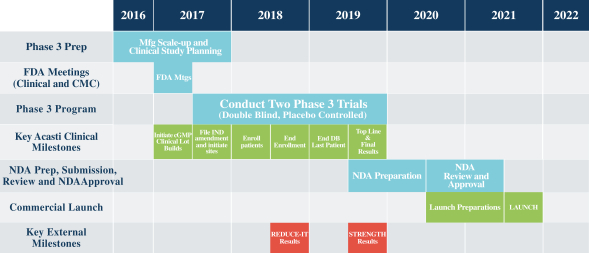
As of the date of this prospectus, we plan to start clinical site activation by the end of 2017.
Our Intellectual Property Strategy
Under a license agreement we entered into with Neptune in August 2008, which we refer to as the license agreement, we received an exclusive license to use Neptunes intellectual property portfolio related to cardiovascular pharmaceutical and medical food applications. The license agreement confers to us freedom-to-operate in order to develop and commercialize CaPre and our novel and active pharmaceutical ingredients, or APIs, for the prescription drug and medical food markets. We entered into the license agreement with Neptune in order to allow us to develop and commercialize CaPre until these Neptune patents expire. Upon the expiry of the last-to-expire licensed Neptune patents in 2022, and the concurrent expiry of our license agreement with Neptune, we believe that CaPre will be fully covered under our own issued and pending patents, and we do not believe that we will afterwards require any license from Neptune or any other third parties to support the commercialization of CaPre.
As a result of a royalty prepayment transaction we entered into with Neptune on December 4, 2012, we are no longer required to pay any royalties to Neptune under the license agreement during its term for the use of the licensed intellectual property. The license agreement expires on the date of the last to expire patent, which is in 2022.
On August 8, 2017, Neptune announced that it sold its krill oil inventory and intellectual property to Aker. Aker then licensed the intellectual property back to Neptune. The license agreement between us and Neptune remains in place and unchanged.
- 54 -
Table of Contents
The following table summarizes the patent applications related to our license agreement with Neptune.
| Patent description | US Patent # | Expiration date of patent |
Holder | |||||||||
| Composition of Matter |
US8,030,348 | (1) | 2022 | Aker | ||||||||
| (NATURAL PHOSPHOLIPIDS OF MARINE ORIGIN CONTAINING FLAVONOIDS AND POLYUNSATURATED PHOSPHOLIPIDS AND THEIR USES) |
||||||||||||
| Method of Use for Dyslipidemia |
US8,057,825 | 2022 | Aker | |||||||||
| (KRILL AND/OR MARINE EXTRACTS FOR PREVENTION AND/OR TREATMENT OF CARDIOVASCULAR DISEASES, ARTHRITIS, SKIN CANCER, PREMENSTRUAL SYNDROME, DIABETES AND TRANSDERMAL TRANSPORT) |
||||||||||||
| Method of Extraction |
US6,800,299 | 2019 | Aker | |||||||||
| (METHOD OF EXTRACTING LIPIDS FROM MARINCE AND AQUATIC ANIMAL TISSUE) |
||||||||||||
| (1) | Three continuations also stem from U.S. Pat. 8,030,348 (U.S. Pat. 8,278,351; and 8,383,675). |
In addition to the license agreement, we continue to expand our own intellectual property, or IP, portfolio and patents. We have now filed patent applications in 24 jurisdictions, including Europe, North America, Asia and Australia for our Concentrated Therapeutic Phospholipid Composition to treat HTG, and we currently have 20 issued or allowed patents and 15 patent applications pending. During the three-month period ended June 30, 2017, additional patents were granted to us by the Taiwanese and Australian patent offices to protect both composition of matter and methods of treatment. The last to expire of our patents is valid until 2031. In August 2017, the South Korean Patent Office has found the patent application (KR 10-2012-7013588) acceptable for grant.
| Patent description | WO (PCT) application # & U.S. Patent # |
Expiration date of patent family |
Number of patents worldwide |
|||
| Method of Use |
US8,586,567 and 9,475,830 |
20* | ||||
| Composition of Matter CONCENTRATED THERAPEUTIC PHOSPHOLIPID COMPOSITION and Method of Use |
WO2011050474 | 2031* | (15 patents pending in approx. 24 countries) |
| * | Five Australian innovation patents are valid until 2018, patent (ZL 201080059930.4) granted by the Chinese Patent Office is valid until 2030 and patent (US 9475830) granted by the United States Patent and Trademark Office is valid until 2031. Our Australian patent AU 2010312238 expires in 2030. |
U.S. patents were granted to us covering a method of reducing serum TG levels comprising administering a composition comprising about 66% phospholipid, or PL, (US 8,586,567), and a method of treating HTG comprising administering a composition comprising about 60% PL (US 9,475,830). We later filed a U.S. continuation patent application to pursue claims directed towards a composition encompassing an extract comprising a PL content between about 60% to about 99%. The U.S. patent granted to us covers the use of concentrated therapeutic phospholipid compositions for treating or preventing diseases associated with cardiovascular disease, metabolic syndrome, inflammation and associated diseases, neurodevelopmental diseases, and neurodegenerative diseases, comprising administering an effective amount of a concentrated therapeutic phospholipid composition. Corresponding patents have also been granted in South Africa, China, Japan, Mexico, Taiwan, Saudi Arabia, Panama, and Israel.
- 55 -
Table of Contents
A patent is generally valid for 20 years from the date of first filing. Patent terms can be further extended depending on the jurisdiction to compensate, for example, for regulatory delays during the pre-market approval process.
We believe these patents increase potential commercial opportunities for CaPre, including through possible licensing and partnership opportunities. We are committed to building a global portfolio of patents to ensure long-lasting and comprehensive intellectual property protection and to safeguard potentially valuable market expansion opportunities.
Our patent No. 600167 in New Zealand, which is enforceable until 2030 and relates to a concentrated phospholipid composition comprising 60% PL and method of using the same for treating cardiovascular diseases, has been opposed by BIO-MER Ltd. Our corresponding Australian patent No. 2010312238 was opposed by Enzymotec Ltd., but that opposition has been since been discontinued. The New Zealand patent opposition is in its early stages. In our view, no new prior art has been presented that was not already considered in other jurisdictions, such as in the United States and Japan, where our patents are in force.
We have applied for trademark protection of CaPre, and we are the owner of the trademark BREAKING DOWN THE WALLS OF CHOLESTEROL in Canada and the United States. The trademark CaPre® is registered in the United States, Canada, Australia, China, Japan and Europe. In addition, we also protect our optimization and extraction processes through industrial trade secrets and know-how.
Manufacturing of CaPre
We are developing CaPre as a new chemical entity (which means a novel chemical product protected by patents), and we plan to conduct our Phase 3 program using good manufacturing practices, or cGMP, good clinical practices, or cGCP, and good laboratory practices, or cGLP. The contract manufacturing organizations, or CMOs, selected by us for manufacturing and packaging are all cGMP compliant. Batch sizes of 10 to 12 kilograms of CaPre have already been successfully produced and tested clinically, and we recently scaled up to 100 kg/day to fulfill the clinical product requirements for our Phase 3 program and initial commercial launch.
In preparation for our Phase 3 program, working together with our pharmaceutical CMOs, we have advanced the installation and qualification of the proprietary extraction and purification equipment used to manufacture CaPre. We ran our first engineered production run of CaPre in December 2016 and our first scaled cGMP production lots of CaPre at CordenPharmas Chenôve facility in Dijon, France during the first half of 2017. As of the date of this prospectus, we have completed 2 clinical lots of CaPre for our Phase 3 studies.
The graphic below illustrates the current manufacturing sequence for CaPre:

CaPre is manufactured under strict cGMP as per 21 CFR parts 210 and 211:
| | Developed as a new molecular entity using robust, unique Quality- by- Design manufacturing process |
| | All CMOs are cGMP compliant (manufacturing and packaging sites) |
| | Proprietary process with small footprint (future patents, trade secrets, and know-how) |
| | Scale up completed from 10-15 kg batch size to 100 kg/day |
- 56 -
Table of Contents
Our Business and Commercialization Strategy
Key elements of our business and commercialization strategy include initially obtaining regulatory approval for CaPre in the United States for severe HTG. We do not currently have dedicated in-house sales and marketing personnel and we are evaluating several alternative go-to-market strategies for commercializing CaPre in the United States. Our preferred strategy outside the United States is to commercialize CaPre through regional or country-specific strategic partnerships, and to potentially seek support and funding from each partner for clinical development, registration and commercialization activities. We believe that a late development-stage and differentiated drug candidate like CaPre could be attractive to various global, regional or specialty pharmaceutical companies, and we are taking a targeted approach to partnering and licensing in various geographies.
We recently entered into a non-binding term sheet with a leading China-based pharmaceutical company. Completion of the transaction is subject to further negotiation and execution of a definitive agreement, which once signed would grant an exclusive license to the Chinese pharmaceutical company to commercialize CaPre in certain Asian countries, including China. If a definitive agreement is reached and signed, the term sheet contemplates that we would receive an upfront payment of US$8 million upon signing, plus potential regulatory and commercial milestone payments in excess of US$125 million, and tiered double-digit royalties on net sales. The term sheet contemplates that the term of the license, including the period during which milestone payments, if any, could be achieved, would be the later of (i) the fifth anniversary of the last-to-expire patent or (ii) 2035, and the license would be automatically renewable for one renewal term of ten years. The term sheet is preliminary and non-binding at this stage and the license, upfront payment, possible milestone payments and royalties contemplated by it will only become operative if definitive documents are executed. It is possible that no definitive agreement will be reached, or, if a definitive agreement is reached, that its terms and conditions may differ from those described above.
If we reach commercialization of CaPre, as part of our sales and marketing strategy, we expect to focus our U.S. launch initially on lipid specialists, cardiologists and primary care physicians who comprise the top prescribers of lipid-regulating therapies for patients with severe HTG.
Our key commercialization goals include:
| | completing our Phase 3 program and, assuming the results are positive, filing an NDA to obtain regulatory approval for CaPre in the United States, initially for the treatment of severe HTG, with the potential to afterwards expand CaPres indication to the treatment of high TGs; |
| | continuing to strengthen our patent portfolio and other intellectual property rights; |
| | continuing to evaluate and determine the optimal strategic approach for commercializing CaPre in the United States; and |
| | pursuing strategic opportunities outside of the United States, such as licensing or similar transactions, joint ventures, partnerships, strategic alliances or alternative financing transactions, to provide development capital, market access and other strategic sources of capital for us. |
In addition to completing our Phase 3 program, we expect that additional time and capital will be required to complete the filing of an NDA to obtain FDA pre-market approval for CaPre in the United States, and to complete business development collaborations, marketing and other pre-commercialization activities before reaching the commercial launch of CaPre.
Competition
The biotechnology and pharmaceutical industries are highly competitive. There are many pharmaceutical companies, biotechnology companies, public and private universities and research organizations actively engaged in the research and development of products that may be similar to CaPre. We believe that the number of companies seeking to develop products and therapies similar to CaPre will likely increase, particularly if the CV outcome trials by Amarin and/or Astra Zeneca are successful.
- 57 -
Table of Contents
Our competitors in the United States and globally include large, well-established pharmaceutical companies, specialty pharmaceutical sales and marketing companies, and specialized cardiovascular treatment companies. GlaxoSmithKline plc, which currently sells LOVAZA, a prescription-only OM3 fatty acid indicated for patients with severe HTG, was approved by the FDA in 2004 and has been available in the U.S. market since 2005. Multiple generic versions of LOVAZA are now available in the United States. Amarin launched its prescription-only OM3 drug VASCEPA in 2013, and reached a market share of approximately 20% by the end of 2015. In addition, EPANOVA (OM3-carboxylic acids) capsules, a free fatty acid form of OM3 (comprised of 55% EPA and 20% DHA), is FDA-approved for patients with severe HTG. Omtryg, another OM3 fatty acid composition developed by Trygg Pharma AS, received FDA approval for severe HTG. Neither EPANOVA nor Omtryg have yet been commercially launched, but could launch at any time. Other large companies with products that would compete indirectly with CaPre include AbbVie, Inc., which currently sells Tricor and Trilipix for the treatment of severe HTG, and Niaspan, which is primarily used to raise HDL-C but is also used to lower TGs. Generic versions of Tricor, Trilipix, and Niaspan are also now available in the United States. In addition, we are aware of a number of other pharmaceutical companies that are developing products that, if approved and marketed, would compete with CaPre.
Raw Materials
We use semi-refined raw krill oil as our primary raw material to produce CaPre. Krill is generally harvested in Antarctic waters. The total quantity of the krill species is estimated to be at least 500,000,000 metric tons. The krill biomass is the worlds most abundant biomass and is monitored to help ensure sustainable cultivation. Historically, we have sourced all of our krill oil from Neptune. On August 8, 2017, Neptune announced its near-term plan to discontinue krill oil production and the sale of its krill oil inventory and intellectual property to Aker. We are evaluating alternative krill oil sources. We have sufficient krill oil inventories that we anticipate will be required to complete our Phase 3 program and we believe that alternative supplies of krill oil that can meet our specifications will be readily available.
Employees, Specialized Skills and Knowledge
Our management consists of professionals from business development, sales and marketing, clinical development, pharmaceutical manufacturing, finance and science backgrounds. Our research team includes scientists with expertise in pharmaceutical development, chemistry, manufacturing and controls, nonclinical and clinical studies, pharmacology, regulatory affairs, quality assurance/quality control, intellectual property and strategic alliances. As of December 20, 2017, we employed 19 people in Canada and the United States, eight of whom have advanced biology, engineering, chemistry, biochemistry or microbiology degrees. We generally require all of our employees to enter into invention assignment, non-disclosure and non-compete agreements. We rely, in part, on some administrative and general accounting support from Neptune, and we also rely on third-party consultants from time to time. Our employees are not covered by any collective bargaining agreement or represented by a trade union.
Additional Information About Our Phase 2 Clinical Trials
Our COLT Trial
Our COLT clinical trial, which was completed in 2014, was a randomized, open-label, dose-ranging, multi-center trial in Canada designed to assess the safety and efficacy of CaPre in the treatment of patients with TG levels between 200-877 mg/dL. The primary objectives of the COLT study were to evaluate the safety and efficacy of 0.5 grams, 1 gram, 2 grams and 4 grams of CaPre per day in reducing fasting plasma TGs over 4 and 8 weeks, as compared to the standard of care alone.
The secondary objectives of the COLT study were to evaluate:
| | the effect of CaPre on fasting plasma TGs in patients with TGs between 200-499 mg/dL (high TGs); |
- 58 -
Table of Contents
| | the dose dependent effect on fasting plasma TGs in patients with TGs between 500-877 mg/dL (severe HTG); and |
| | the effect of CaPre on fasting plasma levels of LDL-C (direct measurement), HDL-C, non-HDL-C, hs-CRP and OM3 index. |
The final results of the COLT trial indicated that CaPre was safe and effective in reducing TGs in patients with mild to severe HTG with significant mean (average) TG reductions above 20% after 8 weeks of treatment with daily doses of 4 grams and 2 grams. Demographics and baseline characteristics of the patient population were balanced in terms of age, race and gender. A total of 288 patients were enrolled and randomized and 270 patients completed the study, which exceeded our targeted number of evaluable patients. From this patient population, approximately 90% had high TGs.
The proportion of patients treated with CaPre that experienced one or more adverse events in the COLT trial was similar to that of the standard of care group (30.0% versus 34.5%, respectively). A substantial majority of adverse events were mild (82.3%) and no severe treatment-related adverse effects were reported. Only one patient was discontinued from the study due to an adverse event of moderate intensity. While the rate of gastrointestinal side effects was higher in the CaPre groups compared to standard of care alone and appeared to increase in a dose-related manner, none of the subjects participating in the study suffered from a serious adverse event. The COLT study results showed that even at higher doses, CaPre is safe and well tolerated with only transient and predominantly mild adverse events occurring at low rates.
The COLT trial met its primary objective of showing CaPre to be safe and effective in reducing TGs in patients with mild to severe HTG. After only a 4-week treatment, CaPre achieved a statistically significant TG reduction as compared to standard of care alone. Standard of care could be any treatment physicians considered appropriate in a real-life clinical setting and included lifestyle modifications as well as statins and/or ezetimibe. Patients treated with 4 grams of CaPre per day over 4 weeks reached a mean TG decrease of 15.4% from baseline and a mean improvement of 18.0% over the standard of care. Results also showed increased benefits after 8 weeks of treatment, with patients on a daily dose of 4 grams of CaPre registering a mean TG decrease of 21.6% from baseline and a mean improvement of 14.4% over the standard of care.
After 8 weeks of treatment, patients treated with 1 gram of CaPre for the first 4 weeks of treatment and 2 grams for the following 4 weeks, showed a statistically significant TG mean improvement of 16.2% over the standard of care, corresponding to a 23.3% reduction for the 1-2 grams patient population as compared to a 7.1% reduction for the standard of care. After 8 weeks of treatment, patients treated with 2 grams of CaPre for the entire 8 weeks showed statistically significant TG mean improvements of 14.8% over the standard of care, corresponding to a 22.0% reduction for the 2 grams as compared to a 7.1% reduction for the standard of care. Also, after 8 weeks of treatment, patients treated with 4 grams for the entire 8 weeks showed statistically significant TG, non-HDL-C and HbA1C mean improvements of 14.4% and 9.8% and 15.0%, respectively, as compared to standard of care. The 4 grams group showed mean improvements in:
| | TGs of 14.4%, corresponding to a reduction of 21.6% as compared to a reduction of a 7.1% for the standard of care group, |
| | non-HDL-C of 9.8%, corresponding to a reduction of 12.0% as compared to a reduction of 2.3% for the standard of care group, and |
| | HbA1c of 15.0%, corresponding to a reduction of 3.5% as compared to an increase of 11.5% for the standard of care group. |
In addition, all combined doses of CaPre showed a statistically significant treatment effect on HDL-C levels, with an increase of 7.4% as compared to standard of care. Trends (p-value < 0.1) were also noted on patients treated with 4 grams of CaPre for the entire 8-week treatment period with mean reduction of total cholesterol of 7.0% and increase of HDL-C levels of 7.7%, as compared to the standard of care. The results of the COLT trial indicated that CaPre has no significant deleterious effect on LDL-C (bad cholesterol) levels.
- 59 -
Table of Contents
Our TRIFECTA Trial
Our TRIFECTA clinical trial, which was completed in 2015, was a 12-week, randomized, placebo-controlled, double-blind, dose-ranging trial in Canada, designed to assess the safety and efficacy of CaPre at a dose of 1 gram or 2 grams on fasting plasma TGs as compared to a placebo in patients with TG levels between 200-877 mg/dL. A total of 387 patients were randomized and 365 patients completed the 12-week study, consistent with our targeted number of evaluable patients. From this patient population, approximately 90% had high TGs with baseline TGs between 200 and 499 mg/dL. The remainder had severe HTG with baseline TGs between 500 and 877 mg/dL. Approximately 30% of patients were on lipid-lowering medications, such as statins, and approximately 10% were diabetic.
Similar to our COLT study, the primary objective of the TRIFECTA study was to evaluate the effect of CaPre on fasting plasma TGs in patients with TGs between 200-877 mg/dL and to assess the tolerability and safety of CaPre. The secondary objectives of the TRIFECTA study were to evaluate:
| | the effect of CaPre on fasting plasma TGs in patients with TGs between 200-499 mg/dL; |
| | the dose dependent effect on fasting plasma TGs in patients with TGs between 500-877 mg/dL; and |
| | the effect of CaPre in patients with high TGs and severe HTG on fasting plasma levels of LDL-C (direct measurement), and on fasting plasma levels of HDL-C, non-HDL-C, hs-CRP and OM3 index. |
CaPre successfully met the TRIFECTAs studys primary objective. The placebo-corrected percentage change in TGs were decreases of 9.1% (p=0.049) and 9.7% (p=0.044) for 1 gram and 2 grams of CaPre, respectively. Key secondary objectives were also met:
| | there was a statistically significant decrease in non-HDL-C versus placebo (p=0.038), with the 2 gram group decreasing by 5.3% from baseline versus placebo over the 12-week period; and |
| | no deleterious effect on LDL-C (bad cholesterol). |
Finally, a statistically significant dose response increase in the OM3 index for patients on 1 gram and 2 grams versus placebo was noted. The OM3 index reflects the percentage of EPA and DHA in red blood cell fatty acids and the risk of cardiovascular disease is considered to be lower as the OM3 index increases.
CaPre was found to be safe and well tolerated at all doses tested, with no serious adverse events that were considered treatment-related. Out of 387 randomized patients, a total of 7 (1.8%) were discontinued as a result of adverse events, three were on placebo, two were on 1 gram and two were on 2 grams. The predominant incidence was gastrointestinal-related, with no difference between CaPre and placebo. The safety profiles of patients on CaPre and placebo were similar.
The COLT and TRIFECTA clinical trials were conducted by JSS Medical Research, a CRO specializing in the pharmaceutical, biotechnology, nutraceutical and medical device industries, which is both owned and managed by Dr. John Sampalis, the brother of Dr. Tina Sampalis, who previously was our President and Chief Global Strategy Officer.
Government Regulation
United States Drug Development
Government authorities in the United States, at the federal, state and local level, and in other countries extensively regulate, among other things, the research, development, testing, manufacture, quality control, approval, labeling, packaging, storage, record- keeping, promotion, advertising, distribution, post-approval monitoring and reporting, marketing and export and import of drug products such as CaPre. Generally, before a new drug can be marketed, considerable data demonstrating its quality, safety and efficacy must be obtained, organized into a format specific to each regulatory authority, submitted for review and approved by the regulatory authority.
- 60 -
Table of Contents
FDA Regulatory Process
In the United States, the FDA regulates drugs under the FDCA and its implementing regulations. Drugs are also subject to other federal, state and local statutes and regulations. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state and local statutes and regulations require the expenditure of substantial time and financial resources.
In order to be marketed in the United States, CaPre must be approved by the FDA through the NDA review process. The process required before a drug may be marketed in the United States generally involves the following:
| | completion of extensive nonclinical (animal) and formulation studies in accordance with applicable regulations, including the FDAs Good Laboratory Practice, or GLP, regulations; |
| | submission of an investigational new drug application, or IND, which must become effective before human clinical trials may begin in the United States; |
| | performance of adequate and well-controlled clinical trials in accordance with the applicable IND and other clinical study- related regulations, such as current Good Clinical Practices, to establish the safety and efficacy of the proposed drug for its proposed indication; |
| | submission of an NDA for a new drug; |
| | satisfactory completion of an FDA pre-approval inspection of the manufacturing facility or facilities where the drug is produced to assess compliance with cGMP to assure that the facilities, methods and controls are adequate to preserve the drugs identity, strength, quality and purity; |
| | satisfactory completion of potential FDA audit of the nonclinical and/or clinical trial sites that generated the data in support of the NDA; and |
| | FDA review and approval of the NDA prior to any commercial marketing or sale of the drug in the United States. |
The data required to support an NDA is generated in two distinct development stages: nonclinical and clinical. The nonclinical development stage generally involves synthesizing or otherwise producing the active component, developing the formulation and determining the manufacturing process, as well as carrying out non-human toxicology, pharmacology and drug metabolism studies in the laboratory, which support subsequent clinical testing. The sponsor must submit the results of the nonclinical tests, together with manufacturing information, analytical data, any available clinical data or literature and a proposed clinical protocol, to the FDA as part of the IND, which is a request for authorization from the FDA to administer an investigational drug product to humans. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA raises concerns or questions regarding the proposed clinical trials. The FDA may also place the IND on clinical hold within that 30-day time period. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. A clinical hold may be imposed at any time before or during a clinical trial due to safety concerns or non-compliance.
The clinical stage of development first involves the administration of the investigational drug to healthy volunteers and then to patients with the disease being targeted with the drug, all done under the supervision of qualified investigators, generally physicians not employed by or under the trial sponsors control, in accordance with cGCP. All research subjects must provide their informed consent for their participation in any clinical trial. Clinical trials are conducted under protocols detailing, among other things, the objectives of the clinical trial, dosing procedures, subject selection and exclusion criteria, data collection, and the parameters to be used to monitor subject safety and assess the investigational drugs efficacy. Each protocol, and any subsequent amendments to the protocol or new investigators information, must be submitted to the FDA as part of the IND. Further, each clinical trial must be reviewed and approved by an independent institutional review board, or IRB, at or servicing each institution at which the clinical trial will be conducted. An IRB is charged with protecting the welfare and rights of trial participants and considers such items as whether the risks to individuals participating
- 61 -
Table of Contents
in the clinical trials are minimized and are reasonable in relation to anticipated benefits. The IRB also approves the informed consent form that must be provided to each clinical trial subject or its legal representative. There are also requirements governing the reporting of ongoing clinical trials and completed clinical trial results to public registries, as well as reporting of safety information under the IND.
Clinical studies are generally conducted in three sequential phases that may overlap, known as Phase 1, Phase 2 and Phase 3 clinical trials. Phase 1 generally involves a small number of healthy volunteers who are initially exposed to a single dose and then multiple doses of the investigational drug. The primary purpose of these studies is to assess the metabolism, pharmacologic action, side effect tolerability and safety of the drug. Phase 2 trials typically involve studies in disease-affected patients to determine the dose required to produce the desired benefits. At the same time, safety and further pharmacokinetic and pharmacodynamic information is collected, as well as identification of possible adverse effects and safety risks and preliminary evaluation of efficacy. Phase 3 clinical trials generally involve large numbers of patients at multiple sites, often in multiple countries (from several hundred to several thousand subjects) and are designed to provide the data necessary to demonstrate the effectiveness of the product for its intended use, its safety in use, and to establish the overall benefit/risk relationship of the product and provide an adequate basis for product approval. Phase 3 clinical trials should, if possible, include comparisons with placebo and may include a comparison to approved therapies. The duration of treatment is often extended to mimic the actual use of a product during marketing. Generally, two adequate and well-controlled Phase 3 clinical trials are required by the FDA for approval of an NDA (Pivotal Studies).
Progress reports detailing the results of the clinical trials must be submitted at least annually to the FDA. In addition, written IND safety reports must be submitted to the FDA and the investigators for serious and unexpected adverse events or any finding from tests in laboratory animals that suggests a significant risk for human subjects. The FDA, the IRB, or the sponsor may suspend or terminate a clinical trial at any time on various grounds, including a finding that the research subjects or patients are being exposed to an unacceptable health risk.
Additionally, some clinical trials are overseen by an independent group of qualified experts organized by the clinical trial sponsor, known as a data safety monitoring board or committee. This group provides oversight and will determine whether or not a trial may move forward at designated check points based on review of interim data from the study. A clinical trial may be terminated or suspended based on evolving business objectives and/or competitive climate.
The manufacturing process must be capable of consistently producing quality batches of the investigational drug and, among other things, must develop methods for testing the identity, strength, quality and purity of the final drug product. The sponsor must develop appropriate labeling that sets forth the conditions of intended use. Additionally, appropriate packaging must be selected and tested and stability studies must be conducted to demonstrate that the drug candidate does not undergo unacceptable deterioration over its shelf life.
Post-approval studies, sometimes referred to as Phase 4 clinical trials, may be conducted after initial marketing approval. These studies are used to gain additional experience from the treatment of patients in the intended therapeutic indication. In certain instances, the FDA may mandate the performance of Phase 4 studies as part of a post-approval commitment, such as pediatric studies.
NDA and FDA Review Process
Nonclinical and clinical information is filed with the FDA in an NDA along with proposed labeling. The NDA is a request for approval to market the drug and must contain proof of safety, purity, potency and efficacy, which is demonstrated by extensive nonclinical and clinical testing. Data may come from company-sponsored clinical trials intended to test the safety and effectiveness of a use of a product, or from a number of alternative sources, including studies initiated by investigators. To support marketing approval, the data submitted must be sufficient in quality and quantity to establish the safety and effectiveness of the investigational drug product to the satisfaction of the FDA.
- 62 -
Table of Contents
The submission of an NDA is subject to the payment of substantial user fees; a waiver of such fees may be obtained under certain limited circumstances. FDA approval of an NDA must be obtained before marketing a drug in the United States. In addition, under the Pediatric Research Equity Act, an NDA or supplement to an NDA must contain data to assess the safety and effectiveness of the drug for the claimed indications in all relevant pediatric subpopulations and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective. The FDA may grant deferrals for submission of data or full or partial waivers.
The FDA reviews all NDAs submitted before it accepts them for filing and may request additional information. The FDA must make a decision on accepting an NDA for filing within 60 days of receipt. Once the submission is accepted for filing, the FDA begins an in-depth review of the NDA. Under the goals and policies agreed to by the FDA under the Prescription Drug User Fee Act, or PDUFA, the FDA has ten months from the filing date in which to complete its initial review of a standard NDA and respond to the applicant. This review typically takes 12 months from the date the NDA is submitted to the FDA including the screening which takes a period of 60 days. The FDA does not always meet its PDUFA goal dates for standard NDAs, and the review process is often significantly extended by FDA requests for additional information or clarification.
After the NDA submission is accepted for filing, the FDA reviews the NDA to determine, among other things, whether the proposed product is safe and effective for its intended use, and whether the product is being manufactured in accordance with cGMP to assure and preserve the products identity, strength, quality and purity. The FDA will likely re-analyze the clinical trial data, which could result in extensive discussions with the FDA.
Before approving an NDA, the FDA will conduct a pre-approval inspection of the manufacturing facilities for the new product to determine whether they comply with cGMP. The FDA will not approve the product unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. In addition, before approving an NDA, the FDA may also audit data from clinical trials to ensure compliance with cGCP requirements. After the FDA evaluates the application, manufacturing process and manufacturing facilities, it will issue a Complete Response Letter, or CRL. A CRL indicates that the review cycle of the application is complete and whether the application is approved and, when applicable, the CRL describes the specific deficiencies in the NDA and may require additional clinical data and/or an additional Phase 3 clinical trial(s), and/or other significant and time-consuming requirements related to clinical trials, nonclinical studies or manufacturing. The applicant may either resubmit the NDA, addressing all of the deficiencies identified in the letter, or withdraw the application. Even if such data and information is submitted, the FDA may ultimately decide that the NDA does not satisfy the criteria for approval.
If a product receives marketing approval, the approval may be significantly limited to specific diseases and dosages or the indications for use may otherwise be limited, which could restrict the commercial value of the product. Further, the FDA may require that certain contraindications, warnings or precautions be included in the product labeling, may condition the approval of the NDA on other changes to the proposed labeling, or may require a Risk Evaluation and Mitigation Strategy (REMS), which could limit the ability to market the drug once approved. The FDA may also require the development of adequate controls and specifications, or a commitment to conduct post-market testing or clinical trials and surveillance to monitor the effects of approved products.
U.S. Post-Marketing Requirements
Following approval of a new product, a pharmaceutical company and the approved product are subject to continuing regulation by the FDA, including, among other things, monitoring and recordkeeping activities, reporting to the applicable regulatory authorities of adverse experiences with the product, providing the regulatory authorities with updated safety and efficacy information, product sampling and distribution requirements, and complying with promotion and advertising requirements, which include, among others,
- 63 -
Table of Contents
standards for direct-to-consumer advertising, restrictions on promoting drugs for uses or in patient populations that are not described in the drugs approved labeling, or off-label use, limitations on industry-sponsored scientific and educational activities, and requirements for promotional activities involving the internet. Although physicians may prescribe legally available drugs for off-label uses, manufacturers and distributors may not market or promote such off-label uses. Modifications or enhancements to the product or its labeling or changes of the site of manufacture are often subject to the approval of the FDA and other regulators, which may or may not be received or may result in a lengthy review process. In some cases, these changes will require the submission of clinical data and the payment of a user fee.
U.S. Patent Term Restoration and Marketing Exclusivity
Depending upon the timing, duration and specifics of the FDA approval of our prescription drug candidates, some of our U.S. patents may be eligible for limited patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984, commonly referred to as the Hatch-Waxman Amendments. The Hatch-Waxman Amendments permit a patent restoration term for an NCE of up to five years as compensation for patent term lost during product development and the FDA regulatory review process for a product that is the first permitted commercial marketed product. However, patent term restoration cannot extend the remaining term of a patent beyond a total of 14 years from the products approval date. The patent term restoration period is generally one-half the time between the effective date of an IND and the submission date of an NDA plus the time between the submission date of an NDA and the approval of that application. Only one patent applicable to an approved drug may be extended and the application for the extension must be submitted prior to the expiration of the patent. The USPTO in consultation with the FDA, reviews and approves the application for any patent term extension or restoration. In the future, we intend to apply for restoration of patent term for one of our currently owned or licensed patents to add patent life beyond its current expiration date, depending on the expected length of the clinical trials and other factors involved in the filing and review of the relevant NDA.
Non-U.S. Drug Regulation
In Canada, biopharmaceutical product candidates are regulated by the Food and Drugs Act and the related rules and regulations, which are enforced by the Therapeutic Products Directorate of Health Canada. In order to obtain approval for commercializing new drugs in Canada, the sponsor must satisfy many regulatory conditions. The sponsor must first complete preclinical studies in order to file a clinical trial application, or CTA, in Canada. The sponsor will then receive different clearance authorizations to proceed with Phase I clinical trials, which can then lead to Phase 2 and Phase 3 clinical trials. Once all three phases of trials are completed, the sponsor must file a registration file named a New Drug Submission, or NDS, in Canada. If the NDS demonstrates that the product was developed in accordance with the regulatory authorities rules, regulations and guidelines and demonstrates favorable safety and efficacy and receives a favorable risk/benefit analysis, then the regulatory authorities issue a notice of compliance, which allows the sponsor to market the product.
In addition to regulations in the United States and Canada, we are subject to a variety of regulations governing clinical studies and commercial sales and distribution of our products in other jurisdictions around the world. These laws and regulations typically require the licensing of manufacturing and contract research facilities, carefully controlled research and testing of product candidates and governmental review and approval of results prior to marketing therapeutic product candidates. Additionally, they require adherence to good laboratory practices, good clinical practices and good manufacturing practices during production. The process of new drug approvals by regulators in the United States, Canada and the European Union are generally considered to be among the most rigorous in the world.
Whether or not the FDA or Health Canada approval is obtained for a product, we must obtain approval from the comparable regulatory authorities of other countries before we can commence clinical studies or marketing of the product in those countries. The approval process varies from country to country and the time may be longer or shorter than that required for the FDA or Health Canada approval. The requirements governing the conduct of
- 64 -
Table of Contents
clinical studies, product licensing, pricing and reimbursement vary greatly from country to country. In some international markets, additional clinical trials may be required prior to the filing or approval of marketing applications within the country.
Active Pharmaceutical Ingredient Regulation
The FDA will regulate finished products containing APIs developed or under development by us. Depending on its intended uses, a finished product containing the API may be regulated as a drug under the procedures described above. It may be possible to market a finished product containing an API developed or under development by us as a dietary supplement. Dietary supplements do not require FDA premarket approval. However, it may be necessary to submit a notification to the FDA that a company intends to market a dietary supplement containing a new dietary ingredient. In general, the regulatory requirements in other countries also depend on the nature of the finished product and do not focus on the API itself.
Property, Plant and Equipment
Our head office and operations are located at 545 Promenade Centropolis, suite 100, Laval, Québec, Canada, H7T 0A3. We do not own our own manufacturing facility for the production of CaPre; however, we do own the proprietary equipment for producing the API and drug product. We currently do not have plans to develop our own manufacturing facility. However, this could change in the foreseeable future, as we consider the most cost-effective approaches to producing CaPre while ensuring the highest level of quality. We currently depend on third party suppliers and manufacturers to produce our required raw krill oil and drug substance and products. If CaPre is approved for distribution by the FDA, we initially expect to rely on cGMP-compliant third parties to manufacture NKPL66, which is the API in CaPre, encapsulate, bottle and package clinical supplies of CaPre.
We have entered into an agreement with CordenPharma Chenôve, a third party CMO, for the manufacturing of CaPre clinical material for the purposes of our Phase 3 program in accordance with cGMP regulations required by the FDA.
Legal Proceedings
Our former CEO is claiming the payment of approximately $8.5 million and the issuance of equity instruments from the Neptune group (including Acasti). As our management believes that these claims are not valid, no provision has been recognized. The Neptune group (including Acasti) also filed an additional claim to recover certain amounts from the former officer.
We are also involved in other matters arising in the ordinary course of our business. Since management believes that all related claims are not valid and it is presently not possible to determine the outcome of these matters, no provisions have been made in our financial statements for their ultimate resolution beyond the amounts incurred and recorded for such matters. The resolution of these other matters could have an effect on our financial statements in the year that a determination is made, however, in managements opinion, the final resolution of all such matters is not projected to have a material adverse effect on our financial position.
- 65 -
Table of Contents
SELECTED FINANCIAL INFORMATION
The following table summarizes our selected historical financial data for the periods, and as of the dates, indicated. Our financial statements have been prepared in accordance with IFRS as issued by the IASB.
We derived the statements of net earnings and comprehensive loss data for the fiscal years ended March 31, 2017, February 29, 2016 and February 28, 2015 and the statement of financial position data as of March 31, 2017 and February 29, 2016 from our audited financial statements and the related notes thereto appearing elsewhere in this prospectus. The statements of net earnings and comprehensive loss data for the fiscal years ended February 28, 2014 and 2013 and the statement of financial position data as at February 28, 2015, 2014 and 2013 are derived from our audited financial statements and the related notes thereto not appearing in this prospectus. We derived the statements of net earnings and comprehensive loss data for the six-month periods ended September 30, 2017 and August 31, 2016 and the statement of financial position data as of September 30, 2017 from our unaudited interim financial statements and the related notes thereto appearing elsewhere in this prospectus. The statement of financial position data as at August 31, 2016 are derived from our unaudited interim financial statements and the related notes thereto not appearing in this prospectus. In the opinion of management, the unaudited interim financial statements reflect all adjustments necessary for a fair presentation of the financial statements. Our historical results are not necessarily indicative of the results that should be expected in the future, and the results for the six-month period ended September 30, 2017 are not necessarily indicative of the results that may be expected for the full year or any other period.
Beginning in fiscal 2017, our fiscal year end is on March 31. Previously, our fiscal year end was February 28. As a result, our financial statements and the corresponding notes to our financial statements appearing elsewhere in this prospectus include a transition year that includes thirteen months of operations, beginning on March 1, 2016 and ending on March 31, 2017, and two different three- and six-month periods: the period ended September 30, 2017 and the period ended August 31, 2016.
You should read this summary financial data together with our financial statements and related notes and the information under Managements Discussion and Analysis of Financial Condition and Results of Operations included elsewhere in this prospectus.
| ($ in thousands, except share and per share |
Six-month period ended | Fiscal year ended | ||||||||||||||||||||||||||
| September 30, 2017 |
August 31, 2016 |
March 31, 2017 |
February 29, 2016 |
February 28, 2015 |
February 28, 2014 |
February 28, 2013 |
||||||||||||||||||||||
| Revenue from sales |
$ | nil | $ | nil | $ | nil | $ | nil | $ | nil | $ | 501 | $ | 724 | ||||||||||||||
| Loss from operating activities |
$ | (7,184 | ) | $ | (5,409 | ) | $ | (11,210 | ) | $ | (9,612 | ) | $ | (12,395 | ) | $ | (10,800 | ) | $ | (6,980 | )(1) | |||||||
| Net loss and total comprehensive loss |
$ | (7,285 | ) | $ | (5,484 | ) | $ | (11,247 | ) | $ | (6,317 | ) | $ | (1,655 | ) | $ | (11,612 | ) | $ | (6,892 | ) | |||||||
| Basic and diluted loss per share |
(0.49 | ) | (0.51 | ) | $ | (1.01 | ) | $ | (0.59 | ) | $ | (0.16 | ) | $ | (1.38 | ) | $ | (0.95 | ) | |||||||||
| Total assets |
$ | 19,757 | $ | 23,552 | $ | 25,456 | $ | 28,517 | $ | 37,208 | $ | 45,632 | $ | 12,170 | ||||||||||||||
| Total liabilities |
$ | 4,951 | $ | 1,540 | $ | 3,753 | $ | 1,297 | $ | 3,980 | $ | 12,352 | $ | 2,446 | ||||||||||||||
| Share capital |
$ | 66,633 | $ | 61,973 | $ | 66,576 | $ | 61,973 | $ | 61,628 | $ | 61,027 | $ | 28,923 | ||||||||||||||
| Total equity |
$ | 14,806 | $ | 22,011 | $ | 21,703 | $ | 27,220 | $ | 33,228 | $ | 33,280 | $ | 9,724 | ||||||||||||||
| Weighted average number of shares outstanding |
14,717,693 | 10,712,038 | 11,094,512 | 10,659,936 | 10,617,704 | 8,436,893 | 7,275,444 | |||||||||||||||||||||
| Dividends declared per share |
| | | | | | | |||||||||||||||||||||
| (1) | The lower loss from operating activities in 2013 compared to later years is in part attributable to an increase in amortization and depreciation following the increase in our license asset as a result of the prepayment of royalties to Neptune in mid-calendar 2013. |
- 66 -
Table of Contents
MANAGEMENTS DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion of our financial condition and results of operations together with the financial statements and the notes thereto included elsewhere in this prospectus. The following discussion contains forward looking statements that reflect our plans, estimates and beliefs. Our actual results could differ materially from those discussed in the forward-looking statements. Factors that could cause or contribute to these differences include those discussed below and elsewhere in this prospectus, particularly those in the section of this prospectus entitled Risk Factors. Our financial statements were prepared in accordance with IFRS as issued by the IASB. See the financial statements included as part of this prospectus and the notes thereto for a discussion of the significant accounting policies and significant estimates and judgments required to be made by management. Our financial results are published in Canadian dollars. All amounts appearing in this MD&A are in thousands of Canadian dollars, except share and per share amounts or unless otherwise indicated.
Overview
We are a biopharmaceutical innovator focused on the research, development and commercialization of prescription drugs using omega 3, or OM3, fatty acids derived from krill oil. OM3 fatty acids have extensive clinical evidence of safety and efficacy in lowering triglycerides, or TGs, in patients with hypertriglyceridemia, or HTG. Our lead product candidate is CaPre, an OM3 phospholipid, which we are developing initially for the treatment of severe HTG, a condition characterized by very high levels of TGs in the bloodstream (> 500 mg/dL). Market research commissioned by us suggests there is a significant unmet medical need for an effective, safe and well-absorbing OM3 therapeutic that demonstrates a positive impact on the major blood lipids associated with cardiovascular disease risk. We believe that, if supported by our Phase 3 program in the United States, which we initiated during the second half of 2017 and for which we plan to start clinical site activation by the end of 2017, CaPre will address this unmet medical need. We also believe the potential exists to expand CaPres initial indication to patients with high TGs (blood levels between 200 499 mg/dL), although at least one additional clinical trial would likely be required to expand CaPres indication to this segment. We may seek to identify new potential indications for CaPre that may be appropriate for future studies and pipeline expansion. In addition, we may also seek to in-license other cardiometabolic drug candidates for drug development and commercialization.
We are subject to a number of risks associated with the conduct of our clinical program and their results, the establishment of strategic alliances and the successful development of new products and their marketing. We are currently not generating any revenues and we have incurred significant operating losses and negative cash flows from operations since inception. To date, we have financed our operations through the public offering and private placement of common shares and convertible debt, the proceeds from research grants and research tax credits, and the exercises of warrants, rights, and options. To achieve the objectives of our business plan, we plan to raise the necessary funds through additional securities offerings and the establishment of strategic alliances as well as additional research grants and research tax credits. CaPre and other product candidates developed by us will require approval from the FDA and equivalent regulatory organizations in other countries before their sale can be authorized. Our ability to ultimately achieve profitable operations is dependent on a number of factors outside of our control.
Our current assets of $5,852 as at September 30, 2017 include cash and cash equivalents totaling $5,329. Our liabilities total $4,951 at September 30, 2017 and are comprised primarily of $3,391 in amounts due to or accrued for creditors, $1,509 for our unsecured convertible debentures and $51 for derivative warrant liabilities. Our positive working capital balance has declined during the current fiscal year and is expected to continue to decline until we raise additional funds or find a strategic partner. Our current assets as September 30, 2017 are projected to be significantly less than needed to support our current liabilities as at that date when combined with the projected level of our expenses for the next twelve months, including the full initiation of, ongoing enrollment of patients in, and manufacturing of clinical materials for, our Phase 3 program for CaPre. Additional funds will also be needed for our expected expenses for our Phase 3 program for CaPre and other needed operations beyond the next twelve months. We also expect to incur certain increased general and administrative expenses as a result of a reduction of our shared administrative services with Neptune, with those added expenses beginning in the
- 67 -
Table of Contents
second quarter of our current fiscal year. We are working towards development of strategic partner relationships and plan to raise additional funds in the near future, but there can be no assurance as to when or whether we will complete any financing or strategic collaborations. If we do not raise additional funds, or find one or more strategic partners we may not be able to realize our assets and discharge our liabilities in the normal course of our business. As a result, there exists a material uncertainty that casts substantial doubt about our ability to continue as a going concern and, therefore, realize our assets and discharge our liabilities in the normal course of business. We currently have no other arranged sources of financing.
Caution Regarding Non-IFRS Financial Measures
We use multiple financial measures for the review of our operating performance. These measures are generally IFRS financial measures, but one adjusted financial measure, Non-IFRS operating loss (adding to net loss, finance expenses, depreciation and amortization and impairment loss, change in fair value of derivative warrant liabilities, stock-based compensation and by subtracting finance income and deferred income tax recovery), is also used to assess our operating performance. We use this measure, in addition to the IFRS financial measures, for the purposes of evaluating our historical and prospective financial performance, as well as our performance relative to competitors and to plan and forecast future periods as well as to make operational and strategic decisions. We believe that providing this Non-IFRS information to investors, in addition to IFRS measures, allows them to see our results through the eyes of our management, and to better understand our historical and future financial performance.
Earnings and other measures adjusted to a basis other than IFRS do not have standardized meanings and are unlikely to be comparable to similar measures used by other companies. Accordingly, they should not be considered in isolation. We use Non-IFRS operating loss to measure our performance from one period to the next without the variation caused by certain adjustments that could potentially distort the analysis of trends in our operating performance, and because we believe it provides meaningful information on our financial condition and operating results. Our method for calculating Non-IFRS operating loss may differ from that used by other corporations.
We calculate our Non-IFRS operating loss measurement by adding to net loss, finance expenses, depreciation and amortization and impairment loss, change in fair value of derivative warrant liabilities, stock-based compensation and by subtracting finance income and deferred tax recovery. Items that do not impact our core operating performance are excluded from the calculation as they may vary significantly from one period to another. Finance income/expenses include foreign exchange gain (loss). We also exclude the effects of certain non-monetary transactions recorded, such as stock-based compensation, from our Non-IFRS operating loss calculation. We believe it is useful to exclude this item as it is a non-cash expense. Excluding this item does not imply it is necessarily non-recurring. A reconciliation of net loss to Non-IFRS operating loss is presented further below.
Basis of Presentation of the Financial Statements
Beginning in fiscal 2017, our fiscal year end is on March 31. Fiscal 2017 is a transition year, and includes thirteen months of operations, beginning on March 1, 2016 and ending on March 31, 2017. As a result, our financial statements and the corresponding notes to our financial statements for the thirteen months of operations beginning on March 1, 2016 and ending on March 31, 2017 include two unaudited periods: the one-month period ended March 31, 2017 and the twelve-month period ended February 28, 2017. In light of the change to our fiscal year end, this MD&A discusses and compares the thirteen-month period ended March 31, 2017, the twelve-month period ended February 29, 2016 and the twelve-month period ended February 28, 2015. In addition, there is a comparative discussion of our results of operations for the three and six-month periods ended September 30, 2017 and August 31, 2016, and a discussion on notable items related to our one-month result of operations ending March 31, 2017.
- 68 -
Table of Contents
Our financial statements have been prepared on a going concern basis, which assumes we will continue our operations in the foreseeable future and will be able to realize our assets and discharge our liabilities and commitments in the ordinary course of business. Our financial statements do not include any adjustments to the carrying values and classification of assets and liabilities and reported expenses that may be necessary if the going concern basis was not appropriate for our financial statements. If we are unable to continue as a going concern, material write-downs to the carrying values of our assets, including the intangible asset, could be required.
Results of Operations
Comparison of the three and six-month periods ended September 30, 2017 and August 31, 2016
The following table summarizes our results of operations for the three and six-month periods ended September 30, 2017 and August 31, 2016:
| Three-month periods ended | Six-month periods ended | |||||||||||||||
| Selected Financial Information |
September 30, 2017 | August 31, 2016 | September 30, 2017 | August 31, 2016 | ||||||||||||
| $ | $ | $ | $ | |||||||||||||
| Net loss |
(4,507 | ) | (2,329 | ) | (7,285 | ) | (5,484 | ) | ||||||||
| Basic and diluted loss per share |
(0.31 | ) | (0.22 | ) | (0.49 | ) | (0.51 | ) | ||||||||
| Non-IFRS operating loss |
(3,423 | ) | (1,625 | ) | (5,519 | ) | (3,911 | ) | ||||||||
| Total assets |
19,757 | 23,552 | 19,757 | 23,552 | ||||||||||||
| Working capital1 |
2,461 | 7,047 | 2,461 | 7,047 | ||||||||||||
| Total non-current financial liabilities |
1,560 | 58 | 1,560 | 58 | ||||||||||||
| Total equity |
14,806 | 22,011 | 14,806 | 22,011 | ||||||||||||
| 1 | The working capital is presented for information purposes only and represents a measurement of our short-term financial health. The working capital is calculated by subtracting current liabilities from current assets. Because there is no standard method endorsed by IFRS requirements, the results may not be comparable to similar measurements presented by other public companies. |
Reconciliation of Net Loss to Non-IFRS Operating Loss
| Three-month periods ended | Six-month periods ended | |||||||||||||||
| September 30, 2017 | August 31, 2016 | September 30, 2017 | August 31, 2016 | |||||||||||||
| $ | $ | $ | $ | |||||||||||||
| Net loss |
(4,507 | ) | (2,329 | ) | (7,285 | ) | (5,484 | ) | ||||||||
| Add (deduct): |
||||||||||||||||
| Stock-based compensation |
295 | 210 | 331 | 275 | ||||||||||||
| Depreciation and amortization |
667 | 615 | 1,334 | 1,223 | ||||||||||||
| Financial expenses (income) |
146 | (55 | ) | 259 | 173 | |||||||||||
| Change in fair value of derivative warrant liabilities |
(24 | ) | (66 | ) | (158 | ) | (98 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Non-IFRS operating loss |
(3,423 | ) | (1,625 | ) | (5,519 | ) | (3,911 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
The net loss totaling $4,507 or ($0.31) per share for the three-month period ended September 30, 2017 increased by $2,178 or ($0.09) per share from the net loss totaling $2,329 or ($0.22) per share for the three-month period ended August 31, 2016. This resulted primarily from the $1,798 increased Non-IFRS operating loss and a $201 increase in financial expense, $42 from a decreased gain due to change in value of the warrant derivative liability, a $85 increase in stock-based compensation and a $52 increase in depreciation and amortization.
- 69 -
Table of Contents
The net loss totaling $7,285 or ($0.49) per share for the six-month period ended September 30, 2017 increased by $1,801 or ($0.02) per share from the net loss totaling $5,484 or ($0.51) per share for the six-month period ended August 31, 2016. This resulted primarily from the $1,608 increased Non-IFRS operating loss and an $86 increase in financial expense, combined with $60 from an increased gain due to the change in value of the warrant derivative liability, a $56 increase in stock-based compensation and a $111 increase in depreciation and amortization.
Stock-based compensation expense increased by $85 to $295 for the three-month period ended September 30, 2017 from $210 for the three-month period ended August 31, 2016. 100,000 options were granted in the three-month period ending September 30, 2017 compared to nil in the three-month period ending August 31, 2016. Stock-based compensation expense increased by $56 to $331 for the six-month period ended September 30, 2017 from $275 for the six-month period ended August 31, 2016. There was an increase of 286,100 options granted in the six-month period ended September 30, 2017 compared to the six-month period ended August 31, 2016. The increase in stock based compensation resulted primarily from the number of options vesting in the comparable periods. At September 30, 2017, 394,346 options were exercisable compared to 197,845 at August 31, 2016.
Depreciation and amortization expense increased by $52 to $667 for the three-month period ended September 30, 2017 from $615 for the three-month period ended August 31, 2016, due to increased operational production equipment. Depreciation and amortization expense increased by $111 to $1,334 for the six-month period ended September 30, 2017 from $1,223 for the six-month period ended August 31, 2016, also due to increased operational production equipment.
Financial expenses increased by $201 to $146 for the three-month period ended September 30, 2017 from income of $55 for the three-month period ended August 31, 2016. This resulted primarily from a $78 change from a foreign exchange gain of $10 for the three-month period ended August 31, 2016 to a foreign exchange loss of $68 for the three-month period ended September 30, 2017. This change also resulted from an increase in interest on convertible debentures of $92 for the three-month period ended September 30, 2017 compared to nil for the three-month period ended August 31, 2016, and a decrease of $33 in interest income and other charges for the three-month period ended September 30, 2017 compared to the three-month period ended August 31, 2016, mainly related to the pledge amount earning interest at 9% that was released by Neptune on September 20, 2016.
Financial expenses increased by $86 to $259 for the six-month period ended September 30, 2017 from $173 for the six-month period ended August 31, 2016. This resulted primarily from a $160 reduced foreign exchange loss from a loss of $264 for the six-month period ended August 31, 2016 to a loss of $104 for the six-month period ended September 30, 2017. This was offset by an increase in interest on convertible debentures of $183 for the six-month period ended September 30, 2017 compared to nil for the six-month period ended August 31, 2016, and a decrease of $76 in interest income and other charges compared to the quarter ended August 31, 2016, mainly related to the pledge amount earning interest at 9% that was released by Neptune on September 20, 2016.
The fair value of the derivative warrant liabilities totaled $51 at September 30, 2017 or $24 less than the $75 fair value at June 30, 2017 and $158 less than the $209 fair value at March 31, 2017. The fair value of the warrants is estimated at each reporting date using the Black-Scholes option pricing model. The fair value of the warrants issued in connection with our previous securities offerings was determined to be $0.58 per warrant upon issuance, $0.03 per warrant at September 30, 2017, $0.04 per warrant at June 30, 2017 and $0.11 per warrant as of March 31, 2017. During the three-and six-month periods ended September 30, 2017, the fluctuation in our stock price and the volatility decline resulted in a gain based on the change in fair value of the warrant liabilities reducing the corresponding liability in the statement of financial position. The fair value of the derivative warrant liabilities totaled $58 at August 31, 2016 or $66 less than the $124 value at May 31, 2016 and $98 less than the $156 fair value at February 29, 2016. In the three and six-month periods ended August 31, 2016, the decline in our stock price and volatility resulted in a gain based on the change in fair value of the warrant liabilities reducing the corresponding liability in the statement of financial position.
- 70 -
Table of Contents
Non-IFRS operating loss increased by $1,798 for the three-month period ended September 30, 2017 to $3,423 compared to $1,625 for the three-month period ended August 31, 2016. This was primarily due to an increase in research and development, or R&D, expenses of $1,642 and an increase in general and administrative, or G&A, expenses of $156, before consideration of stock-based compensation, amortization and depreciation. Non-IFRS operating loss increased by $1,608 for the six-month period ended September 30, 2017 to $5,519 compared to $3,911 for the six-month period ended August 31, 2016. This primarily resulted due to an increase in R&D expenses of $1,152 and an increase in G&A expenses of $456, before consideration of stock-based compensation, amortization and depreciation.
Breakdown of major components of the statement of earnings and comprehensive loss for the three and six-month periods ended September 30, 2017 and August 31, 2016
| Three-month periods ended | Six-month periods ended | |||||||||||||||
| Research and development expenses |
September 30, 2017 | August 31, 2016 | September 30, 2017 | August 31, 2016 | ||||||||||||
| $ | $ | $ | $ | |||||||||||||
| Salaries and benefits |
342 | 248 | 701 | 543 | ||||||||||||
| Stock-based compensation |
90 | 30 | 123 | 42 | ||||||||||||
| Research contracts |
1,553 | 658 | 2,071 | 2,059 | ||||||||||||
| Professional fees |
672 | 63 | 1,042 | 149 | ||||||||||||
| Depreciation and amortization |
667 | 614 | 1,334 | 1,223 | ||||||||||||
| Other |
64 | 4 | 120 | 17 | ||||||||||||
| Government grants and tax credits |
(39 | ) | (23 | ) | (60 | ) | (46 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
3,349 | 1,594 | 5,331 | 3,987 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Three-month periods ended | Six-month periods ended | |||||||||||||||
| General and administrative expenses |
September 30, 2017 | August 31, 2016 | September 30, 2017 | August 31, 2016 | ||||||||||||
| $ | $ | $ | $ | |||||||||||||
| Salaries and benefits |
301 | 229 | 662 | 424 | ||||||||||||
| Administrative fees |
37 | 75 | 88 | 150 | ||||||||||||
| Stock-based compensation |
205 | 181 | 208 | 233 | ||||||||||||
| Professional fees |
405 | 306 | 726 | 478 | ||||||||||||
| Other |
88 | 65 | 169 | 137 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
1,036 | 856 | 1,853 | 1,422 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
Three-month period ended September 30, 2017 compared to three-month period ended August 31, 2016
During the three-month period ended September 30, 2017, we continued to move our R&D program forward as planned on our previously announced timeline for the conduct of our Phase 3 clinical program and production scale-up. The $3,349 in total R&D expenses for the three-month period ended September 30, 2017 totaled $2,592 before depreciation, amortization and stock-based compensation expense, compared to $1,594 in total R&D expenses for the three-month period ended August 31, 2016, or $950 before depreciation, amortization and stock-based compensation expense. This $1,642 increase in R&D expenses before depreciation, amortization and stock-based compensation was mainly attributable to the $895 increase in research contracts as well as an increase of $609 in professional fees. The increased research contract expense resulted primarily from a $693 increase in contracts associated with our clinical trial program, as $956 was incurred primarily with our clinical research organization, or CRO, during the three-month period ended September 30, 2017 in preparation for our Phase 3 clinical study program site activation initiation by the end of 2017. This compares to $263 incurred during the prior comparative period in connection with the completion of contracts under our successful Phase 1 bioavailability bridging clinical study. The remaining $202 in increased research contracts resulted from expanded scale-up production activities relating to CaPre during the three-month period ended September 30,
- 71 -
Table of Contents
2017. The increased professional fees resulted primarily from completing due diligence and preliminary discussions for strategic R&D partnership and licensing arrangements. An increase of $94 in incremental salaries and benefits primarily related to full-time compared to half-time direct leadership and management of R&D, combined with the addition of several technicians to production and quality control during the three-month period ended September 30, 2017 compared to the three-month period ended August 31, 2016.
G&A expenses totaling $831 before stock-based compensation expense for the three-month period ending September 30, 2017 increased by $156 from $675 for the three-month period ended August 31, 2016. This $156 increase was mainly attributable to a $72 increase in salaries and benefits associated with adding full-time executive and managerial headcount to support our strategy and financing while becoming more independent from Neptune, partially offset by a $38 reduction in administrative fees. This increase also resulted from net increased professional fees of $99 due primarily to expenses for legal fees relating to the conduct of our annual and special meeting of shareholders, the completion of our periodic filings and other corporate matters, and the reactivation of our public and investor relations programs. The increased legal fees partially resulted from us becoming more independent from Neptune and resulting increased reliance on external legal counsel. These increases were partially offset by reduced marketing research expenses during the three-month period ended September 30, 2017.
Six-month period ended September 30, 2017 compared to six-month period ended August 31, 2016
As we continued our Phase 3 clinical program progress and production scale-up of CaPre within our R&D program, $5,331 was incurred in total R&D expenses for the six-month period ended September 30, 2017 and $3,874 was incurred before depreciation, amortization and stock-based compensation expense. This compares to $3,987 in total R&D expenses for the six-month period ended August 31, 2016 or $2,722 before depreciation, amortization and stock-based compensation expense. This $1,152 increase in R&D expenses before depreciation, amortization and stock-based compensation was mainly attributable to the $893 increase in professional fees incurred in completing due diligence and preliminary discussions for strategic R&D partnership and licensing arrangements. Research contract expense remained approximately $2,000, but the nature of the expenses changed. Of the $2,000 expenses, $1,059 related to the Phase 3 and other clinical study programs, and $1,011 of contract manufacturing, or CMO, production expenses for the six-month period ended September 30, 2017. This is compared to $1,534 of expenses for our PK Bridging and other clinical study programs and $525 in CMO production expenses for the six-month period ended August 31, 2016. Salary and benefits also contributed to the overall increase by $158 related to R&D management, combined with additional headcount for production and quality control in August 2017, as we advance our Phase 3 clinical study program. Of the increase of $103 in other expenses, $46 related to increased travel expenses for the strategic development due diligence activities.
G&A expenses totaling $1,645 before stock-based compensation expense for the six-month period ending September 30, 2017 increased by $456 from $1,189 for the six-month period ended August 31, 2016. This $456 increase was mainly attributable to a $238 increase in salaries and benefits associated with adding full-time executive and managerial headcount to support our strategy and financing while becoming more independent from Neptune, offset by a $62 reduction in administrative fees. This increase also resulted from increased professional fees of $248 due primarily to expenses relating to reactivating our public and investor relations programs and additional legal fees due to increased independence from Neptune, as well as an increase of $32 in other expenses.
- 72 -
Table of Contents
Comparison of the one-month and thirteen-month periods ended March 31, 2017 and years ended February 29, 2016 and February 28, 2015
The following table summarizes our results of operations for the one-month and thirteen month periods ended March 31, 2017 and fiscal years ended February 29, 2016 and February 28, 2015:
| One-month period ended |
Thirteen-month period ended |
Year ended | Year ended | |||||||||||||
| March 31, 2017 |
March 31, 2017 |
February 29, 2016 |
February 28, 2015 |
|||||||||||||
| $ | $ | $ | $ | |||||||||||||
| Net loss |
(769 | ) | (11,247 | ) | (6,317 | ) | (1,655 | ) | ||||||||
| Basic and diluted loss per share |
(0.05 | ) | (1.01 | ) | (0.59 | ) | (0.16 | ) | ||||||||
| Non-IFRS operating loss |
(406 | ) | (7,798 | ) | (6,569 | ) | (8,507 | ) | ||||||||
| Total assets |
25,456 | 25,456 | 28,517 | 37,208 | ||||||||||||
| Working capital(1) |
8,049 | 8,049 | 10,184 | 18,020 | ||||||||||||
| Total non-current financial liabilities |
1,615 | 1,615 | 156 | 2,357 | ||||||||||||
| Total equity |
21,703 | 21,703 | 27,220 | 33,228 | ||||||||||||
| (1) | Working capital is presented for information purposes only and represents a measurement of our short-term financial health mostly used in financial circles. Working capital is calculated by subtracting current liabilities from current assets. Because there is no standard method endorsed by IFRS requirements, the results may not be comparable to similar measurements presented by other public companies. |
Reconciliation of Net Loss to Non-IFRS Operating Loss
| One-month period ended |
Thirteen-month period ended |
Year ended | Year ended | |||||||||||||
| March 31, 2017 |
March 31, 2017 |
February 29, 2016 |
February 28, 2015 |
|||||||||||||
| $ | $ | $ | $ | |||||||||||||
| Net loss |
(769 | ) | (11,247 | ) | (6,317 | ) | (1,655 | ) | ||||||||
| Add (deduct): |
||||||||||||||||
| Stock-based compensation |
86 | 674 | 309 | 1,553 | ||||||||||||
| Depreciation and amortization/Impairment of intangible assets |
226 | 2,738 | 2,734 | 2,335 | ||||||||||||
| Financial expenses (income) |
29 | 113 | (1,094 | ) | (1,916 | ) | ||||||||||
| Change in fair value of derivative warrant liabilities |
22 | 53 | (2,201 | ) | (8,824 | ) | ||||||||||
| Deferred income tax recovery |
| (129 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Non-IFRS operating loss |
(406 | ) | (7,798 | ) | (6,569 | ) | (8,507 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
The net loss totaling $11,247 or ($1.01) per share for the thirteen-month period ended March 31, 2017 increased $4,930 or ($0.42) per share compared to the net loss totaling $6,317 or ($0.59) per share for the year ended February 29, 2016. This change resulted primarily based on the $1,229 increased non-IFRS operating loss explained below, $2,254 from the increased loss due to the change in value of the warrant derivative liability due to the reduction in our share price, a $1,207 financial expense increase (led by a foreign exchange gain during the prior period transitioning to a foreign exchange loss during the current period), and increased depreciation and stock compensation expense offset by no impairment charge in the current period compared to the $339 charge in the prior period combined with the $129 tax benefit recognized in the current period.
The net loss totaling $6,317 or ($0.59) per share for the year ended February 29, 2016 increased $4,662 or ($0.43) per share compared to the net loss totaling $1,655 or ($0.16) per share for the year ended February 28,
- 73 -
Table of Contents
2015. This change resulted primarily based on the $7,445 decrease in net financial income, including a $6,623 decrease in the fair value of the warrant liabilities and the $810 decrease in the foreign exchange gain offset by the $1,527 decrease in G&A expenses and $1,256 decrease in R&D expenses.
There are no notable matters in stock-based compensation expense and no grants for the one-month period ended March 31, 2017. The overall stock-based compensation expense increased for the thirteen-month period ending March 31, 2017 as a total of 1,300,400 stock options were granted compared to 109,188 stock options being granted for the year ended February 29, 2016. The stock-based compensation expense decreased for the year ended February 29, 2016 compared to the same period in 2015 as the 2012 grants had fully vested.
Depreciation, amortization and impairment expense totaled $2,738 for the thirteen-month period ended March 31, 2017, which approximated the same amount when compared to the year ended February 29, 2016. However, there was a change in the mix of this expense as the thirteen-month period ended March 31, 2017 included only depreciation and amortization with the impact of one additional month of depreciation and amortization expense and the addition of new equipment generating incremental depreciation expense, but not the $339 impairment charge recognized during the year ended February 29, 2016. If the impairment charge is excluded from the expense for the year ended February 29, 2016, then the depreciation and amortization expense totaling $2,395 approximates the expense for the year ended February 28, 2015.
The net financial expenses (income) totaling $29 for the month ended March 31, 2017 also resulted primarily from the interest expense from our recent private placement financing in Canada. Net financial expenses (income) totaling $113 for the thirteen-month period ended March 31, 2017 reflect a $1,207 decrease compared to ($1,094) for the year ended February 29, 2016 primarily resulting from the $1,023 foreign exchange gain recognized during the year ended February 29, 2016 changing to the $180 foreign exchange loss recognized during the thirteen-month period ended March 31, 2017. The foreign exchange changes resulted primarily from the utilization of US$-denominated cash and cash equivalents over the periods generating lower US$-denominated cash and cash equivalents throughout the periods and at March 31, 2017 compared to February 29, 2016 and, the periods then ended combined with a decrease in the reporting US$ exchange rate. The US$-denominated cash, cash equivalents and short-term investments totaled US$3,524 at March 31, 2017 and US$10,314 at February 29, 2016 and the exchange rate reporting of CA$ per US$ was $1.3299 at March 31, 2017 compared to $1.3531 at February 29, 2016. Additionally, interest income for the thirteen-month period ended March 31, 2017 totaled $125 compared to $73 for the year ended February 29, 2016, and $39 in interest expense was incurred in the current period, including $31 in March, in connection with the convertible debentures from the private placement. The net financial expenses (income) of ($1,094) for the year ended February 29, 2016 was $822 less than ($1,916) for the year ended February 28, 2015 based on the lower foreign exchange gain that year.
The fair value of the derivative warrant liabilities totaled $209 at March 31, 2017, or $53 more than the $156 fair value at February 29, 2016, $22 of which was recognized during the one-month ended March 31, 1017. The $156 fair value of the derivative warrant liabilities at February 29, 2016 was $2,201 less than the $2,357 value at February 28, 2015 and the decline in value for the year- ended February 28, 2015 was $8,824. The fair value of the warrants is estimated at each reporting date using the Black-Scholes option pricing model. The fair value of the warrants issued in connection with our previous offerings was determined to be $0.58 per warrant upon issuance, $0.09 per warrant at February 29, 2016 and $0.11 per warrant as of March 31, 2017. In fiscal years 2016 and 2015, the decline in our stock price resulted in gains based on the change in fair value of the warrant liabilities reducing the corresponding liability in the statement of financial position.
We recorded a $129 deferred income tax recovery at February 28, 2017 to reduce to nil an income tax liability that was attributable to the difference between the tax basis and the carrying amount of the unsecured convertible debentures.
The non-IFRS operating loss increased by $1,229 for the thirteen-month period ended March 31, 2017 to $7,798 compared to $6,569 for the year-ended February 29, 2016. This increase was primarily due to the incremental
- 74 -
Table of Contents
one-month period non-IFRS operating loss of $406 for March 2017 as well as increased G&A expenses compared to the prior period before consideration of stock-based compensation and amortization and depreciation. There were no notable matters for the one-month period ended March 31, 2017. The non-IFRS operating loss for the year ended February 29, 2015 totaled $8,507 or a $1,938 decrease compared to the year ended February 29, 2016.
Breakdown of major components of statement of earnings and comprehensive loss for the one-month and thirteen-month periods ended March 31, 2017 and fiscal years ended February 29, 2016 and February 28, 2015
| One-month period ended |
Thirteen-month period ended |
Year ended | Year ended | |||||||||||||
| Research and development expenses |
March 31, 2017 |
March 31, 2017 |
February 29, 2016 |
February 28, 2015 |
||||||||||||
| $ | $ | $ | $ | |||||||||||||
| Salaries and benefits |
104 | 1,294 | 989 | 465 | ||||||||||||
| Stock-based compensation |
18 | 107 | 53 | 258 | ||||||||||||
| Research contracts |
63 | 3,149 | 2,730 | 5,062 | ||||||||||||
| Professional fees |
57 | 634 | 1,171 | 865 | ||||||||||||
| Depreciation and amortization |
226 | 2,738 | 2,395 | 2,335 | ||||||||||||
| Impairment of intangible assets |
| | 339 | | ||||||||||||
| Other |
3 | 61 | 238 | 101 | ||||||||||||
| Government grants and tax credits |
(45 | ) | (330 | ) | (349 | ) | (264 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
426 | 7,653 | 7,566 | 8,822 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| One-month period ended |
Thirteen-month period ended |
Year ended | Year ended | |||||||||||||
| General and administrative expenses |
March 31, 2017 |
March 31, 2017 |
February 29, 2016 |
February 28, 2015 |
||||||||||||
| $ | $ | $ | $ | |||||||||||||
| Salaries and benefits |
110 | 1,198 | 409 | 617 | ||||||||||||
| Administrative fees |
25 | 325 | 579 | 650 | ||||||||||||
| Stock-based compensation |
68 | 567 | 256 | 1,296 | ||||||||||||
| Professional fees |
52 | 1,053 | 616 | 593 | ||||||||||||
| Rent |
10 | 121 | 67 | 99 | ||||||||||||
| Other |
27 | 293 | 119 | 318 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
292 | 3,557 | 2,046 | 3,573 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
Thirteen-month and one-month periods ended March 31, 2017 compared to fiscal year-ended February 29, 2016
R&D expenses totaled $7,653 for the thirteen-month period ended March 31, 2017, or an increase of $87 compared to $7,566 in total R&D expenses for the year ended February 29, 2016. The R&D expense increase resulted primarily from $426 in total R&D expenses during March 2017, the thirteenth month of the period ended March 31, 2017, offset by no intangible asset impairment charge in the thirteen-month period ended March 31, 2017 compared to the $339 charge last year. R&D expenses, before consideration of stock-based compensation, amortization and depreciation and impairments of intangible assets, increased by $29 for the thirteen-month period ended March 31, 2017, including $182 during the month of March 2017, to $4,808 compared to $4,779 for the year ended February 29, 2016. The increase of $29 was mainly attributable to the increase in research contracts of $419 and salaries and benefits of $305, principally offset by decreases in professional fees of $537, other expenses of $177 and government grants of $19. The increase of $419 in research contracts during the thirteen-month period ended March 31, 2017 includes $63 relating to the additional one-month period ended
- 75 -
Table of Contents
March 31, 2017, but was primarily due to the cost of our Phase 2 bioavailability bridging clinical study initiated early in fiscal 2017 exceeding the cost of our other Phase 2 and non-clinical testing completed in fiscal 2016. The increased salaries and benefits represented the cost of the expanded team headcount, led by full-time dedicated management (only part time in prior years), needed for us to continue our pharmaceutical process and analytical development and chemistry manufacturing control scale-up, as planned on our previously announced timeline. The decrease of $537 in professional fees is primarily due to a decrease in the development consulting fees incurred last year for our prior Phase 2 clinical study analytics and the planning for our Phase 2 bridging clinical study during the thirteen-month period ended March 31, 2017.
G&A expenses totaled $3,557 for the thirteen-month period ended March 31, 2017, or an increase of $1,511 compared to total G&A expenses of $2,046 for the year ended February 29, 2016. This period-to-period increase includes $292 in total G&A expenses for the thirteenth month of March 2017, $243 in increased stock-based compensation expense and a $976 increase in other G&A expenses, excluding the thirteenth month and stock-based compensation expenses. G&A expenses, excluding the stock-based compensation, increased $1,200 to $2,990 for the thirteen-month period ended March 31, 2017, including $224 during the month of March 2017, compared to $1,790 for the year ended February 29, 2016. This increase was primarily attributable to a $789 increase in salaries and benefits offset by a $254 decrease in Neptune administrative fees, combined with increased professional fees of $437, rent of $54 and other expenses of $174. The increase in salaries and benefit expenses resulted from our need for the added full-time executive and managerial headcount to lead our strategy, incremental financing and back office while supporting continued and expanded R&D with the need for full-time leadership from our management (which was only part time in prior years). The increased professional fees were principally comprised of expenses associated with our investor and public relations program, the achievement of business development milestones, increased market research expenses, and non-recurring project legal and accounting fees associated with the year-end change and immigration-related fees for the U.S.-resident executives.
Fiscal year ended February 29, 2016 compared to fiscal year ended February 28, 2015
R&D expenses totaled $7,566 for the year ended February 29, 2016, or $1,256 less than $8,822 in total R&D expenses for the year ended February 28, 2015. This R&D expense decrease resulted primarily from R&D expenses, before consideration of stock-based compensation, amortization and depreciation and impairment of intangible assets, decreasing by $1,450 to $4,779 from $6,229. This decrease is mainly attributable to a significant decrease in contract expenses related to our clinical studies of $2,332 and government grants increase of $85, partially offset by an increase in salaries and benefits of $524, professional fees of $306 and other expenses of $137.
G&A expenses totaled $2,046 for the year ended February 29, 2016, or $1,527 less than $3,573 for the year ended February 28, 2015. This G&A expense decrease resulted primarily from G&A expenses, before consideration of stock-based compensation, decreasing by $487 to $1,790 for the year ended February 29, 2016 from $2,277 for the year ended February 28, 2015. This decrease is mainly attributable to decreases in salaries of $208, administrative fees of $71, rent of $32 and other expenses of $199 partially offset by an increase in professional fees of $23.
Selected Quarterly Financial Data
Six-months ended September 30, 2017 and Fiscal year ended March 31, 2017
| (In thousands of dollars) |
Three-month period ended September 30, 2017 |
Three-month period ended June 30, 2017 |
March 31, 2017(1) |
November 30, 2016 |
August 31, 2016 |
May 31, 2016 |
||||||||||||||||||
| $ | $ | $ | $ | $ | $ | |||||||||||||||||||
| Net loss |
(4,507 | ) | (2,778 | ) | (3,367 | ) | (2,397 | ) | (2,330 | ) | (3,154 | ) | ||||||||||||
| Basic and diluted loss per share |
(0.31 | ) | (0.19 | ) | (0.28 | ) | (0.22 | ) | (0.22 | ) | (0.29 | ) | ||||||||||||
| (1) | This fiscal quarter represents a period of four months ended March 31, 2017. |
- 76 -
Table of Contents
Fiscal year ended February 29, 2016
| (In thousands of dollars) |
February 29, 2016 |
November 30, 2015 |
August 31, 2015 |
May 31, 2015 |
||||||||||||
| $ | $ | $ | $ | |||||||||||||
| Net loss |
(1,919 | ) | (2,191 | ) | (1,241 | ) | (966 | ) | ||||||||
| Basic and diluted loss per share |
(0.18 | ) | (0.21 | ) | (0.12 | ) | (0.09 | ) | ||||||||
The increase in net loss, net loss per share and non-IFRS operating loss in the fourth quarter of 2017 can partially be explained by the inclusion of the additional month in comparison to the comparative three-month quarterly financial data. The month of March 2017 explains an increase in the fourth quarter net loss of $769 or ($0.05) per share as well as an increase in non-IFRS operating loss of $406. The variances in net loss from quarter to quarter are mainly due to the changes in fair value of the warrant liabilities, notably for the quarter ended May 31, 2015 with a gain of $1,708, as well as variations in foreign exchange gains or losses, particularly for the quarter ended August 31, 2015 with a foreign exchange gain of $890. The quarterly year-to-year non-IFRS operating loss variances are mainly attributable to fluctuations in research and development expenses from quarter-to-quarter as well as an increase in general and administrative expenses over the prior year in the last three quarters of fiscal 2017.
Liquidity and Capital Resources
Share capital structure
Our authorized share capital consists of an unlimited number of Class A (which we refer to in this prospectus as our common shares), Class B, Class C, Class D and Class E shares, without par value. Our issued and outstanding fully paid shares, stock options, restricted shares units and warrants, were as follows as at September 30, 2017, March 31, 2017, February 28, 2017 and February 29, 2016:
| September 30, 2017 | March 31, 2017 | February 28, 2017 | February 29, 2016 | |||||||||||||
| Class A shares, voting participating and without par value |
14,735,937 | 14,702,556 | 10,712,038 | 10,644,440 | ||||||||||||
| Stock options granted and outstanding |
2,402,188 | 1,424,788 | 454,151 | 429,625 | ||||||||||||
| Restricted share units granted and outstanding |
| | | | ||||||||||||
| 2017 Public offering warrants exercisable at $2.15, until February 21, 2022 |
1,965,259 | 1,965,259 | | | ||||||||||||
| Series 2017 BW Broker warrants exercisable at $2.15, until February 21, 2018 |
234,992 | 234,992 | | | ||||||||||||
| Series 2017 unsecured convertible debentures conversion option contingent warrants exercisable at $1.90, until February 21, 2020(1) |
1,052,630 | 1,052,630 | | | ||||||||||||
| Series 8 warrants exercisable at $15 USD, until December 3, 2018(2) |
1,840,000 | 1,840,000 | 1,840,000 | 1,840,000 | ||||||||||||
| Series 9 warrants exercisable at $13.30 until December 3, 2018 |
161,654 | 161,654 | 161,654 | 161,654 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total fully diluted shares |
22,392,660 | 21,381,879 | 13,167,843 | 13,075,719 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| (1) | The debentures are convertible into common shares at a fixed price of $1.90 per common share, except if we pay before the maturity all or any portion of the convertible debentures. If we pay all or any portion of the convertible debenture before maturity, then warrants become exercisable at $1.90 per common share for the equivalent convertible debenture amount prepaid. |
| (2) | Total of 18,400,000 warrants. In order to obtain one common share, 10 warrants must be exercised for a total amount of US$15.00. |
- 77 -
Table of Contents
Sources of liquidity
We are currently not generating any revenues and we have incurred significant operating losses and negative cash flows from operations since inception. To date, we have financed our operations through the public offering and private placement of common shares and convertible debt, the proceeds from research grants and research tax credits, and the exercises of warrants, rights, and options. To achieve the objectives of our business plan, we plan to raise the necessary funds through additional securities offerings and the establishment of strategic alliances as well as additional research grants and research tax credits. CaPre and any other product candidates developed by us will require approval from the FDA and equivalent regulatory organizations in other countries before their sale can be authorized. Our ability to ultimately achieve profitable operations is dependent on a number of factors outside of our control.
Our current assets of $5,852 as at September 30, 2017 include cash and cash equivalents totaling $5,329. Our liabilities total $4,951 at September 30, 2017 and are comprised primarily of $3,391 in amounts due to or accrued for creditors, $1,509 for our unsecured convertible debentures and $51 for derivative warrant liabilities. Our positive working capital balance has declined during the current fiscal year and is expected to continue to decline until we raise additional funds or find a strategic partner. Our current assets as of this date are projected to be significantly less than needed to support our current liabilities as of that date when combined with the projected level of our expenses for the next twelve months, including not only the preparation for, but the planned initiation of our Phase 3 program for CaPre. Based on a conservative estimate, we believe that the net proceeds of this offering, together with our existing cash and cash equivalents, will enable us to fund our operating expenses and capital expenditure requirements through May 2018. We have based this estimate on assumptions that may prove to be wrong, and we could use our capital resources sooner than we currently expect. Additional funds will also be needed for our expected expenses for our Phase 3 program for CaPre and other needed operations beyond the next twelve months. We are working towards development of strategic partner relationships and plan to raise additional funds in the near future, but there can be no assurance as to when or whether we will complete any financing or strategic collaborations. If we do not raise additional funds, or find one or more strategic partners we may not be able to realize our assets and discharge our liabilities in the normal course of our business. As a result, there exists a material uncertainty that casts substantial doubt about our ability to continue as a going concern and, therefore, realize our assets and discharge our liabilities in the normal course of business. We currently have no other arranged sources of financing.
Cash flows and financial condition
Comparison of the three and six-months periods ended September 30, 2017 and August 31, 2016
Summary
As at September 30, 2017, cash and cash equivalents totaled $5,329 with a use of cash totaling $2,238 for the three-month period and a use of cash totaling $4,443 for the six-month period ended September 30, 2017. This compares to $2,893 in total cash and cash equivalents as at August 31, 2016 with a source of cash totaling $1,502 for the three-month period and a use of cash totaling $134 for the six-month period ended August 31, 2016.
Operating activities
During the three-month periods ended September 30, 2017 and August 31, 2016, our operating activities used cash of $2,060 and $912, respectively, and during the six-month periods ended September 30, 2017 and August 31, 2016, our operating activities used cash of $3,706 and $2,983, respectively, further modified by changes in working capital, excluding cash. The use of cash flows in operating activities for the three and six-month periods ended September 30, 2017 and August 31, 2016 when compared to the net losses for each period are mainly attributable to the change in non-cash operating items, further modified by changes in working capital, excluding cash.
- 78 -
Table of Contents
Investing activities
During the three-month period ended September 30, 2017, our investing activities used cash of $76 compared to generating cash of $2,400 for the three-month period ended August 31, 2016. Cash used by investing activities during the three-month period ended September 30, 2017 was due to the acquisition of equipment of $90, partially offset by interest received of $14. Cash generated by investing activities for the three-month period ended August 31, 2016 was mainly due to the maturity of short-term investments of $3,834, partially offset by the reinvestment of short-term investments of $903 and the acquisition of equipment totaling $542.
During the six-month period ended September 30, 2017, our investing activities used cash of $157 compared to generating cash of $2,915 for the six-month period ended August 31, 2016. Cash used by investing activities during the six-month period ended September 30, 2017 was due to the acquisition of equipment totaling $187, partially offset by interest received of $30. Cash generated by investing activities for the six-month period ended August 31, 2016 was mainly due to the maturity of short-term investments of $13,212, partially offset by a $9,266 reinvestment in short-term investments and the acquisition of equipment totaling $1,053.
Financing activities
During the three-month periods ended September 30, 2017 and August 31, 2016, we used nominal cash in financing activities.
During the six-month period ended September 30, 2017, our financing activities used cash of $422 due primarily to the payment of public offering transaction costs of $381 and the payment of private placement transaction costs of $40 related to securities offerings completed in February 2017. During the six-month period ended August 31, 2016, our financing activities used $15 to pay interest.
See Basis of Presentation for additional discussion of our financial condition, including the need for additional funds and the material uncertainty that casts substantial doubt about our ability to continue as a going concern.
Use of funds
We have used and intend to continue to use the net proceeds from our February 2017 securities offerings to fund our manufacturing scale-up for CaPre and the clinical and regulatory preparations necessary to initiate our Phase 3 clinical program site activation for CaPre by the end of 2017, intellectual property expansion, business development activities, G&A expenses, and working capital. Based on our End-of-Phase 2 meeting with the FDA, which took place after the closing of our securities offerings in February 2017, we still expect that most of the more than $1 million in incremental net proceeds raised over the minimum offering amount disclosed in the prospectus for our February 2017 public securities offering in Canada will be used for Phase 3 clinical program pre-site activation preparation based on the plan now being better defined after the FDA meeting, including our plan to conduct two Phase 3 studies of the same 26-week duration instead of the one study with a greater number of patients to be treated with CaPre.
- 79 -
Table of Contents
Financial position
The following table details the significant changes to the statements of financial position as at September 30, 2017 compared to our most recent fiscal year end at March 31, 2017:
| Accounts |
Increase (Decrease) |
Comments |
||||
| Cash and cash equivalents |
(4,443 | ) | See cash flow statement | |||
| Receivable |
37 | Payments received | ||||
| Prepaid expenses |
(23 | ) | Completion of research contracts | |||
| Equipment |
(109 | ) | Acquisition of equipment and amortization | |||
| Intangible asset |
(1,161 | ) | Amortization | |||
| Trade and other payables |
1,197 | Increased accruals and timing of payments | ||||
| Payable to parent corporation |
56 | Timing of payments | ||||
| Derivative warrant liabilities |
(158 | ) | Change in fair value | |||
| Unsecured convertible debentures |
103 | Accretion of interest | ||||
See the statement of changes in equity in our financial statements for the six-month period ended September 30, 2017 found elsewhere in this prospectus for details of changes to the equity accounts since March 31, 2017.
Derivative warrant liabilities
As of September 30, 2017, $51 included in liabilities represents the fair value of warrants issued as part of our previous securities offerings. The warrants issued in connection with the previous offerings are derivative liabilities (derivative warrant liabilities) for accounting purposes due to the currency of the exercise price (US$) being different from our Canadian dollar functional currency. The warrant liabilities will be settled in common shares. The fair value of the warrants issued in connection with the previous offerings was determined to be $0.58 per warrant upon issuance and $0.03 per warrant as of September 30, 2017. The fair value of the warrants is revalued at each reporting date.
Comparisons of the one-month period ended March 31, 2017; thirteen-month period ended March 31, 2017; and fiscal years ended February 29, 2016 and February 28, 2015
Operating activities
During the one-month period ended March 31, 2017, our operating activities used cash of $746, as primarily explained in the non-IFRS operating loss section above. The use of cash flow in operating activities for the one-month period ended March 31, 2017 is mainly attributable to net loss, as explained in the Reconciliation of Net Loss to Non-IFRS Operating Loss section above, further modified by changes in working capital, excluding cash.
During the thirteen-month period ended March 31, 2017 and the years ended February 29, 2016 and February 28, 2015, our operating activities used cash of $6,958, $6,574 and $7,198, respectively, as primarily explained in the Reconciliation of Net Loss to Non-IFRS Operating Loss section above. The use of cash flows in operating activities for the thirteen-month period ended March 31, 2017 and the years ended February 29, 2016 and February 28, 2015 when compared to the net losses for each period are mainly attributable to the change in non-cash operating items, as explained in the Reconciliation of Net Loss to Non-IFRS Operation Loss section above, offset by reductions in working capital, excluding cash.
Investing activities
During the thirteen-month period ended March 31, 2017 and the years ended February 29, 2016 and February 28, 2015, our investing activities generated cash of $6,888, $8,229 and $7,627, respectively. The cash generated by investing activities during the thirteen-month period ended March 31, 2017 was mainly due to the maturity of
- 80 -
Table of Contents
short-term investments of $22,030, offset by reinvestment in short-term investments totaling $12,765 and the acquisition of equipment totaling $2,527. The cash generated by investing activities during the year-ended February 29, 2016 was mainly due to the maturity of short-term investments of $20,437, offset by the reinvestment in short-term investments totaling $11,954 and acquisition of equipment of $276. The cash generated by investing activities during the year-ended February 28, 2015 was mainly due to the maturity of short-term investments of $22,150, offset by the reinvestment in short-term investments totaling $14,478.
Financing activities
During the thirteen-month period ended March 31, 2017, our financing activities generated cash of $6,864 and decreased from the three-month period ending February 28, 2017, as certain transaction costs associated with the financing activities were paid. The cash generated by financing activities during the thirteen-month period ended March 31, 2017 was mainly due to the net proceeds from our public offering in Canada in February 2017 of common shares and warrants of $5,010 and net proceeds from our private placement in Canada in February 2017 of convertible debentures and contingent warrants of $1,872.
Overall, our cash increased by $6,745, $1,716 and by $635, for the thirteen-month period ended March 31, 2017 and the years ended February 29, 2016 and February 28, 2015, respectively. Cash and cash equivalents as at March 31, 2017 totaled $9,772.
Financial position
The following table details the significant changes to our statements of financial position as at March 31, 2017 compared to February 29, 2016:
| Accounts |
Increase (Decrease) |
Comments | ||||||
| Cash and cash equivalents |
6,745 | See cash flow statement | ||||||
| Short-term investments, including restricted investments |
(9,443 | ) | |
Maturity of short-term investments, decrease in investments |
|
|||
| Receivable |
(193 | ) | Payments received | |||||
| Prepaid expenses |
(247 | ) | Completion of research contracts | |||||
| Equipment |
2,594 | |
Acquisition of laboratory and production equipment |
|
||||
| Intangible asset |
(2,517 | ) | Amortization | |||||
| Trade and other payables |
1,000 | |
Increase in expenses and research contracts |
|
||||
| Payable to parent corporation |
(3 | ) | Payment made to parent company | |||||
| Derivative warrant liabilities |
53 | Change in fair value | ||||||
| Unsecured convertible debentures |
1,406 | |
Debt issued in private placement transaction |
|
||||
See the statement of changes in equity in our financial statements for details of changes to the equity accounts from February 29, 2016.
Derivative warrant liabilities
As of March 31, 2017, the amount of $209 included in liabilities represents the fair value of the warrants issued as part of our previous financings. The warrants forming part of the units issued in connection with our previous financings are derivative liabilities for accounting purposes due to the currency of the exercise price (US$) being different from our Canadian dollar functional currency. The warrant liabilities will be settled in common shares. The fair value of the warrants issued in connection with our previous offerings was determined to be $0.58 per warrant upon issuance and $0.11 per warrant as of March 31, 2017. The fair value of the warrants is revalued at each reporting date.
- 81 -
Table of Contents
Contractual Obligations, Off-Balance-Sheet Arrangements and Commitments
As at September 30, 2017, our liabilities total $4,951, of which $3,391 is due within twelve months, $51 relates to a derivative warrant liability that will be settled in common shares and $1,509 of outstanding unsecured convertible debentures. The principal amount of unsecured convertible debentures may be prepaid, in whole or in part, at any time and from time to time, in cash, in our sole discretion. The debentures are convertible into common shares at a fixed price of $1.90 per common share, except if we pay before the maturity all or any portion of the convertible debentures.
A summary of our contractual obligations at September 30, 2017, is as follows:
| Carrying value |
Total contractual cash flows |
1 year or less |
1 to 3 years |
|||||||||||||
| $ | $ | $ | $ | |||||||||||||
| Trade, other payables and due to parent corporation |
3,391 | 3,391 | 3,391 | | ||||||||||||
| Research and development contracts |
2,786 | 2,786 | 2,786 | | ||||||||||||
| Purchase obligation of equipment |
283 | 283 | 283 | | ||||||||||||
| General and Administrative contract |
21 | 21 | 21 | | ||||||||||||
| Unsecured convertible debentures |
1,509 | 2,383 | 160 | 2,223 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
7,990 | 8,864 | 6,641 | 2,223 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
We have no off-balance sheet arrangements.
Research and development agreements
In the normal course of business, we have signed agreements with various suppliers for them to execute R&D projects and to produce certain tools and equipment. We have reserved certain rights relating to these projects.
We initiated R&D projects that are planned to be conducted over the next 12-month period. As at September 30, 2017, of these R&D agreements, an amount of $1,608 is included in Trade and other payables and an amount of $2,786 remains a future commitment.
We have also entered into a contract to purchase production equipment to be used in the manufacturing of the clinical and future commercial supply of CaPre. As at September 30, 2017, of this equipment, an amount of $165 is included in Trade and other payables and an amount of $283 remains a future commitment.
Contingencies
Our former CEO is claiming the payment of approximately $8.5 million and the issuance of equity instruments from the Neptune group (including Acasti). As management believes that these claims are not valid, no provision has been recognized. The Neptune group (including Acasti) has filed a claim to recover certain amounts from the former CEO. All outstanding share-based payments held by the former CEO were cancelled during our fiscal year ended February 28, 2015.
We are also involved in other matters arising in the ordinary course of our business. Since management believes these claims are not valid and it presently is not possible to determine the outcome of these matters, no provisions have been made in our financial statements for their ultimate resolution beyond the amounts incurred and recorded for such matters. The resolution of such matters could have an effect on our financial statements in the year that a determination is made. However, in managements opinion, the final resolution of all such matters is not projected to have a material adverse effect on our financial position.
- 82 -
Table of Contents
Quantitative and Qualitative Disclosures about Market Risks
Credit risk
Credit risk is the risk of a loss if a customer or counterparty to a financial asset fails to meet its contractual obligations. We have credit risk relating to cash, cash equivalents and short-term investments, which we manage by dealing only with highly-rated Canadian financial institutions. The carrying amount of financial assets, as disclosed in the statements of financial position, represents our credit exposure at the reporting date.
Currency risk
We are exposed to the financial risk related to the fluctuation of foreign exchange rates and the degrees of volatility of those rates. Foreign currency risk is limited to the portion of our business transactions denominated in currencies other than the Canadian dollar. Fluctuations related to foreign exchange rates could cause unforeseen fluctuations in our operating results. A portion of our expenses, mainly related to research contracts and purchase of production equipment, is incurred in US dollars and in Euros, for which no financial hedging is required. There is a financial risk related to the fluctuation in the value of the US dollar and the Euro in relation to the Canadian dollar. In order to minimize the financial risk related to the fluctuation in the value of the US dollar in relation to the Canadian dollar, funds continue to be invested as short-term investments in the US dollar. A significant portion of our cash and cash equivalents are denominated in US dollars, further exposing us to fluctuations in the value of the US dollar in relation to the Canadian dollar. See Note 19 of our audited annual financial statements found elsewhere in this prospectus.
Interest rate risk is the risk that the fair value or future cash flows of a financial instrument will fluctuate because of changes in market rates. As at September 30, 2017, March 31, 2017, February 28, 2017 and February 29, 2016, our cash and cash equivalents and our short term investments were subject to fluctuations in short-term fixed interest rates.
Our capacity to reinvest the short-term amounts with equivalent return will be impacted by variations in short-term fixed interest rates available on the market. Management believes the risk we will realize a loss as a result of the decline in the fair value of its short-term investments is limited because these investments have short-term maturities and are generally held to maturity. Our capacity to reinvest the short-term amounts with equivalent return will be impacted by variations in short-term fixed interest rates available on the market. Management believes the risk we will realize a loss as a result of the decline in the fair value of our short-term investments is limited because these investments have short-term maturities and are generally held to maturity.
Liquidity risk
Liquidity risk is the risk that we will not be able to meet our financial obligations as they fall due. We manage liquidity risk through the management of its capital structure and financial leverage, as outlined in Note 22 to our annual financial statements found elsewhere in this prospectus. We also manage liquidity risk by continuously monitoring actual and projected cash flows. Our board of directors reviews and approves our operating budgets, and reviews material transactions outside the normal course of business. Our contractual obligations related to financial instruments and other obligations and liquidity resources are presented in Liquidity and Capital Resources.
Use of Estimates and Measurement of Uncertainty
The preparation of the financial statements in conformity with IFRS requires our management to make judgments, estimates and assumptions that affect the application of accounting policies and the reported amounts of assets, liabilities, income and expenses. Actual results may differ from these estimates. Estimates are based on managements best knowledge of current events and actions that we may undertake in the future. Estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in
- 83 -
Table of Contents
the period in which the estimates are revised and in any future periods affected. Critical judgments in applying accounting policies that have the most significant effect on the amounts recognized in the financial statements include identification of triggering events indicating that the intangible assets might be impaired and the use of the going concern basis of preparation of the financial statements. At the end of each reporting period, management assesses the basis of preparation of the financial statements. The financial statements have been prepared on a going concern basis in accordance with IFRS. The going concern basis of presentation assumes that we will continue our operations for the foreseeable future and can realize our assets and discharge our liabilities and commitments in the normal course of business. Assumptions and estimation uncertainties that have a significant risk of resulting in a material adjustment within the next financial year include determination of the recoverable amount of our cash generating unit, or CGU, and measurement of derivative warrant liabilities and stock-based compensation. Also, management uses judgment to determine which research and development, or R&D, expenses qualify for R&D tax credits and in what amounts. We recognize the tax credits once we have reasonable assurance that they will be realized. Recorded tax credits are subject to review and approval by tax authorities and therefore, could be different from the amounts recorded.
Critical Accounting Policies
Impairment of non-financial assets
The carrying value of our license asset is reviewed at each reporting date to determine whether there is any indication of impairment. If any such indication exists, then the CGUs recoverable amount is estimated. The identification of impairment indicators and the estimation of recoverable amounts require the use of judgment.
Derivative warrant liabilities
The warrants forming part of the units issued in our public offering are derivative liabilities for accounting purposes due to the currency of the exercise price being different from our functional currency. The derivative warrant liabilities are required to be measured at fair value at each reporting date with changes in fair value recognized in earnings. We use Black-Scholes pricing model to determine the fair value. The model requires the assumption of future stock price volatility, which is estimated based on weighted average historic volatility. Changes to the expected volatility could cause significant variations in the estimated fair value of the derivative warrant liabilities.
Stock-based compensation
We have a stock-based compensation plan, which is described in note 15 of our annual financial statements and elsewhere in this prospectus. We account for stock options granted to employees based on the fair value method, with fair value determined using the Black-Scholes model. The Black Scholes model requires certain assumptions such as future stock price volatility and expected life of the instrument. Expected volatility is estimated based on weighted average historic volatility. The expected life of the instrument is estimated based on historical experience and general holder behavior. Under the fair value method, compensation cost is measured at fair value at date of grant and is expensed over the awards vesting period with a corresponding increase in contributed surplus. For stock options granted to non-employees, we measure based on the fair value of services received, unless those are not reliably estimable, in which case we measure the fair value of the equity instruments granted. Compensation cost is measured when we obtain the goods or the counterparty renders the service.
Tax credits
Refundable tax credits related to eligible expenses are accounted for as a reduction of related costs in the year during which the expenses are incurred as long as there is reasonable assurance of their realization.
- 84 -
Table of Contents
Future accounting changes
A number of new standards, interpretations and amendments to existing standards were issued by the IASB, or the IFRS Interpretations Committee, or IFRIC, that are mandatory but not yet effective for the thirteen-month and one-month periods March 31, 2017 and have not been applied in preparing our financial statements. The following standards have been issued by the IASB with effective dates in the future that have been determined by management to impact the financial statements:
Financial instruments
On July 24, 2014, the IASB issued the final version of IFRS 9, Financial Instruments, which addresses the classification and measurement of financial assets and liabilities, impairment and hedge accounting, replacing IAS 39, Financial Instruments: Recognition and Measurement. IFRS 9 is effective for annual periods beginning on or after January 1, 2018, with earlier adoption permitted. We intend to adopt IFRS 9 in our financial statements for the annual period beginning on April 1, 2018. We have not yet assessed the impact of adoption of IFRS 9, and do not intend to early-adopt IFRS 9 in our financial statements.
Amendments to IFRS 2Classification and Measurement of Share-Based Payment Transactions
On June 20, 2016, the IASB issued amendments to IFRS 2, Share-Based Payment, clarifying how to account for certain types of share-based payment transactions. The amendments apply for annual periods beginning on or after January 1, 2018. Earlier application is permitted. As a practical simplification, the amendments can be applied prospectively. Retrospective, or early application is permitted if information is available without the use of hindsight. The amendments provide requirements on the accounting for the effects of vesting and non-vesting conditions on the measurement of cash-settled share-based payments; share-based payment transactions with a net settlement feature for withholding tax obligations; and a modification to the terms and conditions of a share- based payment that changes the classification of the transaction from cash-settled to equity-settled. We intend to adopt the amendments to IFRS 2 in our financial statements for the annual period beginning on April 1, 2018. We have not yet assessed the impact of adoption of the amendments of IFRS 2, and do not intend to early-adopt these amendments in our financial statements.
- 85 -
Table of Contents
BOARD OF DIRECTORS AND MANAGEMENT
Directors and Senior Management
The following table sets out the name and the province or state and country of residence of each of our directors and all offices with us held by them, their principal occupation and the year in which they became a director.
| Name, province or state and country of |
Principal occupation |
First year as director | ||||
| Roderick N. Carter |
Principal, Aquila Life Sciences LLC | 2015 | ||||
| California, United States |
||||||
| Chairman of the Board |
||||||
| Jean-Marie (John) Canan |
Corporate Director | 2016 | ||||
| Florida, United States |
||||||
| Janelle DAlvise |
President and CEO of Acasti | 2016 | ||||
| California, United States |
||||||
| Richard P. Schottenfeld |
Managing Partner & CEO of Schottenfeld Group, LLC | 2017 | ||||
| New York, United States |
||||||
| Katherine Crewe |
Chair, TEC Canada | 2017 | ||||
| Quebec, Canada |
||||||
The business address for our directors and senior management is 545 Promenade du Centropolis, Suite 100, Laval, Québec, Canada H7T 0A3.
The following is a brief biography of our directors and senior management:
Dr. Roderick N. Carter
Dr. Carter has a strong history of contributions to healthcare through clinical, research, business and people leadership. He has significant experience developing and commercializing nutraceutical and pharmaceutical products and has successfully led clinical research and business development strategies for cardiovascular and inflammation related diseases. Dr. Carter is currently Principal at Aquila Life Sciences LLC, a consulting firm he founded in April 2008 focusing on pharmaceutical development and commercialization. Prior to this, he was Vice President of Clinical Development at Reliant Pharmaceuticals, which developed the OM3 cardiovascular drug LOVAZA, and today is a wholly-owned subsidiary of GlaxoSmithKline. He also served as Executive Director at Merck and Co., USA, President and Chief Executive Officer of WellGen and Senior Medical Director at Pfizer Inc., USA. Dr. Carter received his Medical Degree from the University of Witwatersrand, Johannesburg, along with a Master of Science degree in Sports Medicine from Trinity College, Dublin.
Jean-Marie (John) Canan
Mr. Canan is an accomplished business executive with over 34 years of strategic, business development and financial leadership experience. Mr. Canan recently retired from Merck & Co., Inc. where his last senior position was as Senior Vice-President, Global Controller, and Chief Accounting Officer for Merck from November 2009 to March 2014. He has managed all interactions with the audit committee of the Merck board of directors, while participating extensively with the main board and the compensation & benefits committee. Mr. Canan serves as a director of REV Group, a public company, where he chairs the audit committee. Mr. Canan also provides consulting services to Willow BioPharma, a Canadian start-up, engaged in the acquisition and development of legacy pharmaceutical assets. He also serves on the board of trustees of Angkor Hospital for Children, where he also chairs the audit & risk committee. Mr. Canan is a graduate of McGill University, Montreal, Canada, and is a Canadian Chartered Accountant.
- 86 -
Table of Contents
Janelle DAlvise (also our CEO)
Ms. DAlvise has extensive experience the life science industry, having held executive positions in companies commercializing pharmaceuticals, diagnostics, medical devices, and drug discovery research tools. Prior to joining Acasti in mid-2016, Ms. DAlvise was the President and Chairman of Pediatric Bioscience. Before that, she was the CEO of Gish Biomedical, a cardiopulmonary medical device company. Prior to Gish, Ms. DAlvise was the CEO of the Sidney Kimmel Cancer Center (SKCC), a drug discovery research institute, the Co-Founder/President/CEO/Chairman of NuGEN, Inc., and the Co-Founder and Executive VP/COO of Metrika Inc. Ms. DAlvise built NuGEN and Metrika from technology concept through to successful regulatory approvals, product introduction and sustainable revenue growth. Prior to her experience as an entrepreneur, Ms. DAlvise held the positions of VP of Drug Development at Syntex/Roche and Business Unit Director of their Pain and Inflammation business, and VP of Commercial Operations at SYVA (Syntexs clinical diagnostics division). She began her career with Diagnostic Products Corporation where she ran global sales, marketing and service. Ms. DAlvise has a B.S. in Biochemistry from Michigan Technological University. She has completed post-graduate work at the University of Michigan, Stanford University, and the Wharton Business Schools. Ms. DAlvise has served on the board of numerous private companies and non-profits, and is an Entrepreneur-in-Residence for the von Liebig Institute for Entrepreneurship at the University of California, San Diego.
Richard P. Schottenfeld
Mr. Schottenfeld is the founder and Chairman of Schottenfeld Group Holding, the parent company of Koyote Capital which is a proprietary trading firm in New York City, U.S.A. He has also served as the general partner of Schottenfeld Associates and the Schottenfeld Opportunity Fund. Mr. Schottenfeld currently also serves as a non-executive director of Neptune. Mr. Schottenfeld is a graduate of Franklin & Marshall College with degrees in both Economics and Government. Mr. Schottenfeld has been a frequent guest on CNBC and other business news programs.
Katherine Crewe, ICD.D
Ms. Crewe has spent 30 years in the medical device and pharmaceutical manufacturing space for companies with sales and distribution networks spanning the globe. During her career, she held several executive positions in various operations and quality management positions, most recently as Managing Director, Canadian operations, at Mallinckrodt Pharmaceuticals. Ms. Crewe is currently Chair of TEC Canada, and currently also serves as a non-executive director of Neptune. Ms. Crewe holds a Master of Engineering (Biomedical) from McMaster University and a Bachelor of Science (Chemical Engineering) from Queens University.
Linda P. OKeefeChief Financial Officer (CFO) and Secretary
Ms. OKeefe has been our Chief Financial Officer since November 27, 2016 and our Secretary since August 30, 2017. She has worked with both public and private biotechnology, diagnostics, medical devices and healthcare services firms, and also in other private equity-financed markets, including business services, education and technology. Prior to joining us, Ms. OKeefe consulted with various firms after serving as Chief Financial Officer and executive-in-residence for Gryphon Investors, a San Francisco-based private equity firm. At Gryphon Investors, she led fundraising, limited partner relations, risk management and advised portfolio company management teams on growth, financing and back office strategies. In addition, Ms. OKeefe provided mergers & acquisitions and integration support, established and led audit committees, and supported the expansion of teams and systems to meet the needs of growing companies. Ms. OKeefe also served as Chief Financial Officer of Delphi Ventures, a healthcare-focused venture capital firm, and Elevate Ventures; as Vice President of Finance at Genelabs Technologies and Target Therapeutics; and as Controller at Collagen Corporation. Ms. OKeefe is an active Certified Public Accountant and Chartered Global Management Accountant in California and Indiana and was formerly an audit senior with Ernst & Young. She is a member of the American Institute of CPAs, the California and Indiana Societies of CPAs, Association for Corporate Growth, Financial Executives International, and Healthcare Financial Management Association. Ms. OKeefe holds a Bachelor of Science in Business from the University of California, Berkeley.
- 87 -
Table of Contents
Dr. Pierre LemieuxChief Operating Officer (COO)
Dr. Lemieux has been our Chief Operating Officer since April 12, 2010. Previously, Mr. Lemieux was CEO, Co-Founder and Chairman of BiolActis Inc. which he sold in 2009 to interests affiliated with the Nestlé multinational group. Mr. Lemieux joined Suprateck Pharma in 1999 as Director and Vice-President involved in the development of formulations for gene therapy on behalf of Rhone-Poulenc Rorer and Genzyme, which today are under the Sanofi banner. Prior to this, Mr. Lemieux was involved in the development of cardiovascular products at Angiotech Pharmaceuticals. Mr. Lemieux has a Ph.D. in biochemistry from Université Laval (Québec). He holds 16 patents and has authored over 50 publications. Mr. Lemieuxs research was conducted at Université Laval as well as at the anti-cancer center Paul Papin DAngers (France) and the University of Nottingham (England). His research focused on ovarian cancer and its treatment with monoclonal antibodies used to target cancer drugs. After completing his graduate studies, Mr. Lemieux joined the Oncology division of the Center for Health Research, University of Texas (U.S.). He obtained a postdoctoral fellowship from the Susan G. Komen Foundation (Breast Cancer). Mr. Lemieux has served on the boards of BioQuébec, Montreal in vivo and PharmaBio Development.
Mr. Laurent HarveyVice President, Clinical and Non-Clinical Affairs
Mr. Harvey has more than 25 years experience in the biopharmaceutical industry, primarily in drug development and clinical research. Before joining us, he occupied different management positions at Bristol-Myers Squibb, Æterna-Zentaris, Innodia, Bellus Health and KLOX Technologies. During his career, he participated in many national and international clinical programs in various therapeutic fields such as cardiovascular, endocrinology, oncology and neurology. Mr. Harvey holds a Bachelors degree in pharmacy and M.Sc. in hospital pharmacy, both from Université de Montréal.
GHR Committee
The mandate of our governance & human resources, or GHR, committee consists of the evaluation of the proposed nominations of senior executives and director candidates to our board of directors, recommending for board approval, if appropriate, revisions of our corporate governance practices and procedures, developing new charters for any new committees established by the board of directors, monitoring relationships and communication between management and the board of directors, monitoring emerging best practices in corporate governance and oversight of governance matters and assessing the board of directors and its committees. The board of directors receives recommendations from the GHR committee, but retains responsibility for managing its own affairs by, among other things, giving its approval for the composition and size of the board of directors, and the selection of candidates nominated for election to the board of directors. The GHR committee initially evaluates candidates for nomination for election as directors, having regard to the background, employment and qualifications of possible candidates.
The GHR committee is also in charge of establishing the procedure which must be followed by us to comply with applicable guidelines of the TSXV and NASDAQ Stock Market regarding corporate governance. The board of directors does not have a nominating committee and has not adopted any formal written director term limit policy.
Compensation of our executive officers and directors is recommended to the board of directors by the GHR committee. In its review process, the GHR committee relies on input from management on the assessment of executives and corporate performance. As of the date of this prospectus, the GHR committee is composed of the following members, each of whom is independent within the meaning of NI 52-110 and NASDAQ Stock Exchange rules: Dr. Carter, acting as interim chairperson, Ms. Crewe and Mr. Canan. The GHR committee establishes management compensation policies and oversees their general implementation. All members of the GHR committee have direct experience which is relevant to their responsibilities as GHR committee members. All members are or have held senior executive or director roles within significant businesses, several also having
- 88 -
Table of Contents
public companies experience, and have a good financial understanding which allows them to assess the costs versus benefits of compensation plans. The members combined experience in our sector provides them with the understanding of our success factors and risks, which is very important when determining metrics for measuring success.
Audit Committee
Our audit committee is responsible for assisting the board of directors in fulfilling its oversight responsibilities with respect to financial reporting, including:
| | reviewing our procedures for internal control and management performing financial functions; |
| | reviewing and approving the engagement of the auditor; |
| | reviewing annual and quarterly financial statements and all other material continuous disclosure documents, including our annual information form and managements discussion and analysis; |
| | assessing our financial and accounting personnel; |
| | assessing our accounting policies; |
| | reviewing our risk management procedures; and |
| | reviewing any significant transactions outside our ordinary course of business and any pending litigation involving us. |
The audit committee has direct communication channels with our management performing financial functions and our external auditor to discuss and review such issues as the audit committee may deem appropriate. As of August 15, 2017, the audit committee is composed of Mr. Canan, as chairperson, Dr. Carter and Mr. Schottenfeld. Each is financially literate and independent within the meaning of NI 52-110 and the Exchange Act. Our board of directors has determined that Mr. Canan is an audit committee financial expert, as defined by applicable regulations of the U.S. Securities and Exchange Commission. The Commission has indicated that the designation of Mr. Canan as an audit committee financial expert does not make him an expert for any purpose, impose any duties, obligations or liability on Mr. Canan that are greater than those imposed on members of the audit committee and board of directors who do not carry this designation or affect the duties, obligations or liability of any other member of the audit committee or board of directors.
Code of Business Conduct and Ethics
The board of directors adopted a Code of Business Conduct and Ethics, or Code of Conduct, for our directors, officers and employees on May 31, 2007, as amended from time to time. Our Code of Conduct can be found on SEDAR at www.sedar.com and on our web site on www.acastipharma.com. A copy of the Code of Conduct can also be obtained by contacting our Corporate Secretary. Since its adoption by the board of directors, any breach of the Code of Conduct must be brought to the attention of the board of directors by our CEO or other senior executives. No report has ever been filed which pertains to any conduct of a director or executive officer that constitutes a breach to our Code of Conduct.
Since the adoption of the Code of Conduct and the following policies, the board of directors actively monitors compliance with the Code Conduct and promotes a business environment where employees are encouraged to report malfeasance, irregularities and other concerns. The Code of Conduct provides for specific procedures for reporting non-compliant practices in a manner which, in the opinion of the board of directors, encourages and promotes a culture of ethical business conduct.
The board of directors also adopted a disclosure policy, insider trading policy, majority voting policy, management and board compensation policies, and a whistleblower policy.
- 89 -
Table of Contents
In addition, under the Civil Code of Québec, to which we are subject as a legal person incorporated under the Business Corporations Act (Québec) (L.R.Q., c. S-31), a director must immediately disclose to the board any situation that may place him or her in a conflict of interest. Any such declaration of interest is recorded in the minutes of proceeding of the board of directors. The director abstains, except if required, from the discussion and voting on the question. In addition, it is our policy that an interested director recuse himself or herself from the decision-making process pertaining to a contract or transaction in which he or she has an interest.
Director Independence
Our board of directors believes that, in order to maximize its effectiveness, the board must be able to operate independently. A majority of directors must satisfy the applicable tests of independence, such that the board of directors complies with all independence requirements under applicable corporate and securities laws and stock exchange requirements applicable to us. No director will be independent unless the board of directors has affirmatively determined that the director has no material relationship with us or any of our affiliates, either directly or indirectly or as a partner, shareholder or officer of an organization that has a relationship with us or our affiliates. Such determinations will be made on an annual basis and, if a director joins the board of directors between annual meetings, at such time.
The board of directors determined that Mr. Canan, Dr. Carter, Ms. Crewe and Mr. Schottenfeld are independent within the meaning of NI 52-110 and NASDAQ Stock Market rules.
The board of directors determined that Ms. DAlvise is not independent within the meaning of NI 52-110 and NASDAQ given that she is our President and CEO.
Compensation
Summary of our Compensation Programs
Our executive compensation program is intended to attract, motivate and retain high-performing senior executives, encourage and reward superior performance and align the executives interests with ours by providing compensation which is competitive with the compensation received by executives employed by comparable companies and ensuring that the achievement of annual objectives is rewarded through the payment of bonuses and providing executives with long-term incentive through the grant of stock options.
Our GHR committee has authority to retain the services of independent compensation consultants to advise its members on executive compensation and related matters, and to determine the fees and the terms and conditions of the engagement of those consultants. During our fiscal year ended March 31, 2017, the GHR committee retained compensation consulting services, including those led by Lockton Companies, to review our executive compensation programs, including base salary, short-term and long-term incentives, total cash compensation levels and total direct compensation of certain senior positions, against those of peer groups of similar and larger size, as measured by market capitalization, biotechnology and pharmaceutical companies listed or headquartered in North America. All of the services provided by the consultants were provided to the GHR committee. The GHR committee assessed the independence of the consultants and concluded that its engagement of the consultants did not raise any conflict of interest with us or any of our directors or executive officers. As influenced by the consultants fiscal period 2017 executive compensation review, the board and GHR committee set the following executive compensation program.
For executives, more than half of target direct compensation (base salary + target STIP awards + target LTIP awards) is considered at risk. We believe this mix results in a strong pay-for-performance relationship and an alignment with shareholders and is competitive with other firms of comparable size in similar fields. The CEO (or any person acting in that capacity) makes recommendations to the GHR committee as to the compensation of our executive officers, other than himself or herself, for approval by the board. The GHR committee makes
- 90 -
Table of Contents
recommendations to the board of directors as to the compensation of the CEO, for approval. The CEOs salary is based on comparable market consideration and the GHR committees assessment of his or her performance, with regard to our financial performance and progress in achieving strategic goals.
Risk management is a primary consideration of the GHR committee when implementing its compensation program. We do not believe that our compensation program results in unnecessary or inappropriate risk taking, including risks that are likely to have a material adverse effect on us. Payments of bonuses, if any, are not made unless performance goals are met.
Qualitative factors beyond the quantitative financial metrics are also a key consideration in determination of individual executive compensation payments. How executives achieve their financial results and demonstrate leadership consistent with our values are key to individual compensation decisions.
The following table sets forth the compensation information for the named executive officers during the thirteen months ended March 31, 2017, and the fiscal years ended February 29, 2016 and February 28, 2015.
| Name and principal position |
Period ended |
Salary ($) |
Share- based awards(1)(2) ($) |
Option- based awards(1)(2) ($) |
Annual incentive plans ($) |
All other compensation ($)(3) |
Total compensation ($) |
|||||||||||||||||||
| Janelle DAlvise(4) |
March 31, 2017 |
365,072 | | 502,163 | 136,049 | (6) | | 1,003,284 | ||||||||||||||||||
| CEO |
||||||||||||||||||||||||||
| Linda P. OKeefe(5) |
March 31, 2017 |
114,183 | | 237,340 | 39,897 | (7) | 109,414 | (8) | 500,834 | |||||||||||||||||
| CFO |
||||||||||||||||||||||||||
| Pierre Lemieux COO |
March 31, 2017 |
275,819 | | 96,522 | 49,000 | | 421,341 | |||||||||||||||||||
| February 29, 2016 |
239,565 | | 33,320 | 42,000 | | 314,885 | ||||||||||||||||||||
| February 28, 2015 |
202,115 | | 22,163 | 12,000 | | 236,278 | ||||||||||||||||||||
| Laurent Harvey |
March 31 2017 |
194,846 | | 84,205 | 35,000 | | 314,051 | |||||||||||||||||||
| Vice President, Clinical and Non- Clinical Affairs |
||||||||||||||||||||||||||
| February 29, 2016 |
159,808 | | 17,153 | 16,000 | | 192,961 | ||||||||||||||||||||
| February 28, 2015 |
107,977 | | 7,388 | 8,000 | | 123,365 | ||||||||||||||||||||
| (1) | We have adopted IFRS 2 Share-Based Payment to account for the issuance of stock options to employees and non-employees. The fair value of stock options is estimated at the grant date using the Black-Scholes option pricing model. This model requires the input of a number of parameters, including share price, share exercise price, expected share price volatility, expected time until exercise and risk-free interest rates. Although the assumptions used reflect managements best estimates, they involve inherent uncertainties based on market conditions generally outside of our control. |
| (2) | The fair value of the option-based awards granted in the thirteen-month period ended March 31, 2017 is as follows: (i) the May 12, 2016 option- based awards are based on a fair value of $0.96 per option granted to Ms. DAlvise; (ii) the May 30, 2016 option-based awards are based on a fair value of $1.18 per option granted to Dr. Lemieux and Mr. Harvey; (iii) the February 24, 2017 option-based awards are based on a fair value of $1.19 per option granted to Ms. OKeefe and Dr. Lemieux and Mr. Harvey. For the period ended on February 29, 2016, the fair market value of the June 1, 2015 option-based awards are based on a fair value of $1.97 per option granted to Messrs. Harvey and Lemieux. |
- 91 -
Table of Contents
For the period ended on February 28, 2015, the fair market value of the October 20, 2014 option-based awards granted to Dr. Lemieux is based on a fair value of $3.00 per option, prior to our reverse share split.
| (3) | The value of perquisites and other personal benefits received by these executives did not total an aggregate value of $50,000 or more, and does not represent 10% or more of their total salary during the financial years ended March 31, 2017, February 29, 2016 and February 28, 2015. |
| (4) | Ms. DAlvise was appointed our President and CEO on May 11, 2016 and began her functions on June 1, 2016. Her employment agreement provides for payments in U.S. dollars with an annual base salary of US$330,000. |
| (5) | Ms. OKeefe was appointed our CFO effective as of November 27, 2016. Her employment agreement provides for payments in U.S. dollars with an annual base salary of US$250,000. |
| (6) | US$102,300, converted as at March 31, 2017, based on a closing exchange rate of US1.00= $1.3299. |
| (7) | US$30,000 converted as at March 31, 2017, based on a closing exchange rate of US1.00= $1.3299. |
| (8) | Consulting services from July 2016 to November 2016 which provided for payments in U.S. dollars: US$82,273, converted as at March 31, 2017 based on a closing exchange rate of US1.00= $1.3299. |
Short Term Incentive Plan (STIP)
Our Short-Term Incentive Plan, or STIP, provides for potential rewards when a threshold of corporate performance is met. Personal objectives that support corporate goals are established annually with each employee and are assessed at the end of each financial year. Personal objectives are assessed through a performance grid, with pre-specified, objective performance criteria. STIP awards are paid out in proportion to individual performance, determined in end-of-year performance reviews. For the most senior participants in the STIP, greater weight is assigned to corporate objectives. Target payout is expressed as a percentage of base salary and is determined by employment contracts and board discretion. Annual salary for STIP purposes is the annual salary in effect at the end of the plan year (i.e., prior to annual salary increases).
The actual amount awarded ranges from zero for performance well below expectation and is capped at two times target for exceptional performance. The STIP is a discretionary variable compensation plan and all STIP payments are subject to board approval. Participants must be employed by us at the end of the financial year to qualify. We reserve the right to modify or discontinue the STIP at any time.
Ms. DAlvise, our CEO, is eligible for up to a 50% bonus of her annual base salary and Ms. OKeefe, our CFO, is eligible for up to a 40% bonus of her annual base salary. Dr. Lemieux, our COO, is eligible for up to a 40% bonus of his annual base salary and Mr. Harvey, Vice President, Clinical and Non-Clinical Affairs, is eligible for up to a 30% bonus of his annual base salary.
These performance goals will take into account the achievement of R&D milestones within timelines and budget and individual objectives determined annually by the board according to short-term priorities.
Long Term Incentive Plan (LTIP)
The LTIP has been adopted as a reward and retention mechanism. Participation is determined annually at the discretion of the board. Employees approved by our board of directors may participate in our stock option plan, which is designed to align the long-term interests of participants with those of shareholders, in order to promote shareholder value.
The GHR committee determines the number of stock options to be granted to a participant based on peer group data and taking into account corporate performance and level in the organization. The LTIP calculation is based on a guideline percentage of base salary and the number of options is determined based on an approved dollar value (rather than a specific number of shares). The guideline ranges from 15% to 200% and is subject to adjustment by the board in reviewing annual achievement of corporate performance and availability of shares.
- 92 -
Table of Contents
The GHR committee may also determine, in its sole discretion, ad hoc stock option awards to be granted to participants in order to address extraordinary situations. Awards at any level may be adjusted as necessary to maintain an equity burn rate and overhang similar to comparator companies. In addition to our stock option plan, the board is also empowered to grant ad hoc awards, from time to time, under our equity incentive plan to provide for a share-related mechanism to attract, retain and motivate qualified directors, senior employees and consultants.
Our directors and executive officers are not permitted to purchase financial instruments, such as prepaid variable forward contracts, equity swaps, collars or units of exchange funds that are designed to hedge or offset a decrease in market value of equity securities granted as compensation or held, directly or indirectly, by the director or officer.
Stock Option Plan
Our stock option plan was adopted by our board of directors on October 8, 2008 and has been amended from time to time, including most recently on June 14, 2017. The grant of options is part of the long-term incentive component of executive and director compensation and an essential part of compensation. Qualified directors, employees and consultants may participate in our stock option plan, which is designed to encourage optionnees to link their interests with those of our shareholders, in order to promote an increase in shareholder value. Awards and the determination of any exercise price are made by our board of directors, after recommendation by the GHR committee. Awards are established, among other things, according to the role and responsibilities associated with the participants position and his or her influence over appreciation in shareholder value. Any award grants a participant the right to purchase a certain number of common shares during a specified term in the future, after a vesting period and/or specific performance conditions, at an exercise price equal to at least 100% of the market price (as defined below) of our common shares on the grant date. The market price of common shares as of a particular date generally means the closing price per common share on the TSXV, or any other exchange on which the common shares are listed from time to time, for the last preceding date on which there was a sale of common shares on that exchange (subject to certain exceptions set forth in the stock option plan in the event that we are no longer traded on any stock exchange). Previous awards may sometimes be taken into account when new awards are considered.
In accordance with the stock option plan, all of an option holders options will immediately vest on the date of a Change of Control event (as defined in the stock option plan), subject to the terms of any employment agreement or other contractual arrangement between the option holder and us.
However, in no case will the grant of options under the plan, together with any proposed or previously existing security based compensation arrangement, result in (in each case, as determined on the grant date): the grant to any one consultant within any 12-month period, of options reserving for issuance a number of common shares exceeding in the aggregate 2% of our issued and outstanding common shares (on a non-diluted basis); or the grant to any one employee, which provides investor relations services, within any 12-month period, of options reserving for issuance a number of common shares exceeding in the aggregate 2% of our issued and outstanding common shares (on a non-diluted basis).
Options granted under the stock option plan are non-transferable and are subject to a minimum vesting period of 18 months, with gradual and equal vesting on no less than a quarterly basis. They are exercisable, subject to vesting and/or performance conditions, at a price equal to the closing price of the common shares on the TSXV on the day prior to the grant of such options. In addition, and unless otherwise provided for in the agreement between us and the holder, options will also lapse upon termination of employment or the end of the business relationship with us except that they may be exercised for 60 days after termination or the end of the business relationship (30 days for investor relations services employees), to the extent that they will have vested on such date of termination of employment, except in the case of death, disability or retirement where this period is extended to 12 months.
- 93 -
Table of Contents
Subject to the approval of relevant regulatory authorities, including the TSXV, if applicable, and compliance with any conditions attached to that approval (including, in certain circumstances, approval by disinterested shareholders) if applicable, the board of directors has the right to amend or terminate the stock option plan. However, unless option holders consent to the amendment or termination of the stock option plan in writing, any such amendment or termination of the stock option plan cannot affect the conditions of options that have already been granted and that have not been exercised under the stock option plan.
Options for common shares representing a fixed rate of 20% of our outstanding issued common shares as of February 29, 2016 may be granted by the board under the stock option plan. As at the March 31, 2017, there were 657,619 common shares reserved for issuance under the stock option plan. As of March 31, 2017, there were 1,424,788 options outstanding under the stock option plan.
On June 14, 2017, we granted options to certain of our employees, executives and directors under our stock option plan to acquire an aggregate of 647,900 Common Shares at an exercise price of $1.77 per share. The board of directors also amended our stock option plan in order to increase the current limit of shares reserved for issuance under the plan by 798,104 Common Shares to 2,940,511 Common Shares, and approved the grant of options to certain officers and directors to acquire an additional aggregate amount of 373,600 Common Shares at an exercise price of $1.77 per share. At our last annual and special shareholders meeting held on August 15, 2017, disinterested shareholders approved a resolution to approve, ratify and confirm the previous grant of a total of 373,600 options to purchase our common shares to certain of our directors and officers. On August 31, 2017, we granted 100,000 options to the two newly elected directors at an exercise price of $1.60 per share.
On June 14, 2017, the board approved amendments to the existing limits for common shares reserved for issuance under the stock option plan as described below, which were approved by the shareholders at our annual and special shareholders meeting held on August 15, 2017. Our shareholders also approved amendments to the equity incentive plan on August 15, 2017, to set the total number of common shares reserved for issuance pursuant to awards granted under the equity incentive plan to an aggregate number that:
| | if, and for so long as the common shares are listed on the TSXV, will not exceed the lower of: |
| | 367,563 common shares, and |
| | 20% of the issued and outstanding common shares as of March 31, 2017, (equating to 2,940,511 common shares), which number will include common shares issuable pursuant to options issued under the amended stock option plan; or |
| | if, and for so long as the common shares are listed on the TSX, will not exceed 2.5% of the issued and outstanding common shares from time to time. |
Equity Incentive Plan
On May 22, 2013, our equity incentive plan was adopted by the board in order to, among other things, provide us with a share-related mechanism to attract, retain and motivate qualified directors, employees and consultants. The adoption of the equity incentive plan was initially approved by shareholders at our 2013 Shareholders meeting held on June 27, 2013.
Eligible persons may participate in the equity incentive plan. Eligible persons under the equity incentive plan consist of any director, officer, employee or consultant (as defined in the equity incentive plan) of us or a subsidiary. A participant is an eligible person to whom an award has been granted under the equity incentive plan. The equity incentive plan provides us with the option to grant to eligible persons bonus shares, restricted shares, restricted share units, performance share units, deferred share units and other share-based awards.
If, and for so long as our common shares are listed on the TSXV, no more than 2% of the issued and outstanding common shares may be granted to any one consultant or employee conducting investor relations activities in any 12-month period.
- 94 -
Table of Contents
The equity incentive plan is administered by the board and the board has sole and complete authority, in its discretion, to determine the type of awards under the equity incentive plan relating to the issuance of common shares (including any combination of bonus shares, restricted share units, performance share units, deferred share units, restricted shares or other share-based awards) in such amounts, to such persons and under such terms and conditions as the board may determine, in accordance with the provisions of the equity incentive plan and the recommendations made by the GHR committee.
Subject to the adjustment provisions provided for in the equity incentive plan and the applicable rules and regulations of all regulatory authorities to which we are subject (including any stock exchange), the total number of common shares reserved for issuance pursuant to awards granted under the equity incentive plan will be equal to a number that (A) if, and for so long as the common shares are listed on the TSXV, will not exceed either (i) 367,563 common shares, and (ii) 20% of the issued and outstanding common shares as of March 31, 2017, representing 2,940,511 common shares, which includes common shares issuable pursuant to options issued under our stock option plan.
On June 14, 2017, the board approved amendments to the existing limits of common shares reserved for issuance under the stock option plan as described above, which were approved by the shareholders at our annual and special shareholders meeting held on August 15, 2017.
The amendments to the amended stock option plan and amended equity incentive plan are subject to TSXV final approval.
Other Forms of Compensation
RRSP Matching Program. Effective June 1, 2016, we sponsor a voluntary Registered Retirement Savings Plan, or RRSP, matching program, which is open to all eligible employees, including NEOs. The RRSP matching program matches employees contributions up to a maximum of $1,000 per fiscal year for eligible employees who participate in the program. Other than matching contributions under the RRSP matching program (which amounts are disclosed in the column entitled All Other Compensation in the summary compensation table below), we do not provide pension or retirement benefits to our executive officers or directors.
Other Benefits and Perquisites. Our executive employee benefit program also includes life, medical, dental and disability insurance. These benefits and perquisites are designed to be competitive overall with equivalent positions in comparable organizations. We do not have a pension plan for employees.
Compensation of Directors
Our directors compensation consists of an annual fixed compensation of US$35,000. While our compensation structure does not include meeting fees, a discretionary reduction of 20% may be applied to the annual retainer payment each time a director fails to attend a quarterly board or committee session. In addition, the chairman of the board and each chairperson of the audit and the GHR committees received additional compensation of US$25,000 and US$10,000, respectively, for their additional work during the fiscal year ended March 31, 2017. The directors are also entitled to be reimbursed for travelling and other reasonable expenses properly incurred by them in attending meetings of the board or any committee or in otherwise serving us, in accordance with our policy on travel and expenses.
Following their first election to our board of directors, non-executive directors are eligible to receive an initial equity grant of up to 150% of their annual cash retainer worth of stock options vesting annually in equal installments over a 3-year period, subject to the other terms and conditions set forth under the heading Stock Option Plan. In addition to their initial grant, non-executive directors are eligible to receive an annual equity-based award equal to 100% of their total annual cash retainer vesting quarterly in equal installments over an
- 95 -
Table of Contents
18-month period. These awards will be granted at the same time that we are performing our annual performance review for our employees, subject to availability of common shares and subject to the terms and conditions described under the headings Stock Purchase Plan and Equity Incentive Plan. The level of these awards will be consistent with equivalent awards in comparable companies obtained from the benchmark exercise and in accordance with the recommendations obtained from our independent compensation consultant.
The total compensation for our non-executive directors during the thirteen-month period ended March 31, 2017 was as follows:
| Name |
Fees earned ($) |
Option-based awards(1)(2) ($) |
All other compensation ($)(5) |
Total ($) |
||||||||||||
| Roderick N. Carter |
188,517 | (3) | 236,860 | | 425,377 | |||||||||||
| Jean-Marie (John) Canan |
44,884 | (4) | 58,520 | | 103,404 | |||||||||||
| James S. Hamilton |
| | | | ||||||||||||
| Leendert H. Staal(6) |
44,884 | (4) | 58,520 | | 103,404 | |||||||||||
| Pierre Fitzgibbon(6) |
21,917 | | | 21,917 | ||||||||||||
| (1) | We have adopted IFRS 2 Share-Based Payment to account for the issuance of stock options to employees and non-employees. The fair value of the awards is estimated at the grant date using the Black-Scholes option pricing model. This model requires the input of a number of parameters, including share price, share exercise price, expected share price volatility, expected time until exercise and risk-free interest rates. Although the assumptions used reflect managements best estimates, they involve inherent uncertainties based on market conditions generally outside of our control. |
| (2) | For the thirteen-month period ended on March 31, 2017, (i) the fair market value of the May 30, 2016 option-based awards is based on a fair value of $1.18 per option granted to Dr. Carter; and (ii) the fair market value of the February 24, 2017 option-based awards is based on a fair value of $1.17 per option granted to Mr. Canan and Dr. Staal. |
| (3) | Dr. Carter was appointed Executive Chairman of the board on March 1, 2016 and earned compensation of US$98,980 for this role through June 30, 2016. After that date and Ms. DAlvises appointment as CEO on June 1, 2016, Dr. Carter earned compensation of US$45,000 for being Chairman of the board through March 31, 2017. |
| (4) | Mr. Canan and Dr. Staal earned a director compensation of US$33,750, based on a closing exchange rate of US$1.00 = $1.3299 as of March 31, 2017, and were appointed to the board of directors in July 2016. |
| (5) | The directors do not receive pension benefits or other non-equity based annual compensation. |
| (6) | After the resignation of certain directors on February 29, 2016, Mr. Fitzgibbon, Chairman of the board of directors of Neptune, joined until July 12, 2016 as member of our board of directors and Chair of the audit and GHR committees to help insure a proper transition between the departing directors and the election of the new nominees at our 2016 annual general shareholders meeting. |
- 96 -
Table of Contents
Outstanding Share-Based and Option-Based Awards for Directors
The following table provides information about the number and value of the outstanding share-based and option-based awards held by non-executive directors as of March 31, 2017. There were no share-based awards outstanding as of the date of this prospectus.
| Name / grant date | Number of securities underlying unexercised options |
Option exercise price ($)(1) |
Option expiration date |
Value of unexercised in-the-money options ($)(2) |
||||||||||||
| Roderick N. Carter |
||||||||||||||||
| May 30, 2016 |
200,000 | 1.99 | May 29, 2023 | | ||||||||||||
| August 19, 2015 |
10,000 | (1) | 4.80 | August 19, 2022 | | |||||||||||
| Jean-Marie (John) Canan |
||||||||||||||||
| February 24, 2017 |
50,000 | 1.65 | February 24, 2027 | | ||||||||||||
| Leendert H. Staal |
||||||||||||||||
| February 24, 2017 |
50,000 | 1.65 | February 24, 2027 | | ||||||||||||
| (1) | Option-based awards were consolidated following our share consolidation. The exercise price was increased proportionally to reflect the consolidation. |
| (2) | Calculation is based on a trading price of $1.83 for our common shares on the TSXV, as at closing on March 31, 2017. |
None of our share-based and stock options held by non-executive directors that vested during our fiscal year ended on March 31, 2017 were in-the-money at their respective vesting date.
- 97 -
Table of Contents
The following table and accompanying footnotes sets forth, as of December 20, 2017, information regarding beneficial ownership of our common shares by:
| | each person, or group of affiliated persons, known by us to beneficially own more than 5% of our common shares; |
| | each of our executive officers; |
| | each of our directors; and |
| | all of our executive officers and directors as a group. |
Beneficial ownership is determined according to the rules of the SEC and generally means that a person has beneficial ownership of a security if he, she or it possesses sole or shared voting or investment power of that security, including options and warrants that are currently exercisable or exercisable within 60 days of December 20, 2017. Common shares subject to options and warrants currently exercisable or exercisable within 60 days of December 20, 2017 are deemed to be outstanding for computing the percentage ownership of the person holding these options and/or warrants and the percentage ownership of any group of which the holder is a member, but are not deemed outstanding for computing the percentage of any other person.
Except as indicated by the footnotes below, we believe, based on the information furnished to us, that the persons named in the table below have sole voting and investment power with respect to all common shares shown that they beneficially own, subject to community property laws where applicable. The table is based upon information supplied by officers, directors and principal stockholders and Schedule 13Gs filed with the SEC.
Unless otherwise indicated, the address of each beneficial owner listed in the table below is c/o 545 Promenade du Centropolis, Suite 100, Laval, Québec, Canada H7T 0A3.
| Name of beneficial owner |
Number of shares beneficially owned |
Percentage of shares beneficially owned before offering |
Percentage of shares beneficially owned after offering(1) |
|||||||||
| 5% or greater shareholders |
||||||||||||
| Neptune Technologies & Bioressources Inc. |
5,064,694 | 34.0 | % | 20.4 | % | |||||||
| George W. Haywood(2) |
1,479,000 | 9.9 | % | 6.0 | % | |||||||
| Executive officers and directors |
||||||||||||
| Roderick N. Carter |
0 | * | * | |||||||||
| Jean-Marie (John) Canan |
57,499 | * | * | |||||||||
| Katherine Crewe |
0 | * | * | |||||||||
| Richard Schottenfeld |
50,000 | * | * | |||||||||
| Janelle DAlvise |
52,500 | * | * | |||||||||
| Linda P. OKeefe |
30,000 | * | * | |||||||||
| Pierre Lemieux |
7,000 | * | * | |||||||||
| Laurent Harvey |
1 | * | * | |||||||||
| All executive officers and directors as a group (8 persons) |
197,000 | * | * | |||||||||
| (1) | The percentage of common shares beneficially owned after the offering is based on the number of shares outstanding prior to the offering plus the common shares that we are selling in this offering. |
| (2) | Based on information set forth in a Schedule 13G filed with the SEC on March 22, 2017 by George Haywood. The address of Mr. Haywood is c/o Moomjian, Waite & Coleman, LLP, 100 Jericho Quadrangle, Suite 208, Jericho, New York 11753. |
- 98 -
Table of Contents
Our calculation of the percentage of beneficial ownership prior to this offering is based on 14,914,658 common shares issued and outstanding as of December 20, 2017. Beneficial ownership representing less than 1% is denoted with an asterisk (*).
Neptune also owns a warrant entitling it to acquire 592,500 common shares (in order to obtain 1 common share, 10 warrants must be exercised). Our 5% or greater shareholders do not have different voting rights than other holders of our common shares. To the best of our knowledge, there are no other beneficial owners of 5% or more of any class of our voting securities.
- 99 -
Table of Contents
R&D
We are charged by Neptune for the purchase of research supplies and for certain costs incurred by Neptune for our benefit. In the three-month period ended September 30, 2017, we were charged $8,000 for R&D expenses and $93,000 for G&A expenses (for the three-month period ended August 31, 2016, we were charged $9,000 and $132,000, respectively). In the six-month period ended September 30, 2017, we were charged $26,000 for R&D expenses and $196,000 for G&A expenses (for the six-month period ended August 31, 2016, we were charged $9,000 and $258,000, respectively).
In the fiscal year ended March 31, 2017, we were charged $60,000 for R&D expenses and $618,000 for G&A expenses. In the fiscal year ended February 29, 2016, we were charged $371,000 and $790,000, respectively. In the fiscal year ended February 28, 2015, we were charged $344,000 and $876,000, respectively.
Where Neptune incurs specific incremental costs for our benefit, it charges those amounts to us directly. Costs that benefit more than one entity of the Neptune group are charged by allocating a fraction of costs incurred by Neptune that is commensurate to the estimated fraction of services or benefits received by each entity for those items. During the three and six-month period ended September 30, 2017, we recognized an expense of $56,000 and $109,000, respectively, in G&A expenses and nil and $6,000, respectively, in R&D expenses relative to the incremental costs (for the three and six-month period ended August 31, 2016$57,000 and $108,000, respectively, in G&A expenses and nil and nil, respectively, in R&D expenses). We purchased from Neptune R&D supplies totaling $113,000, of which $73,000 as of March 31, 2017, which is recorded in prepaid expenses and will be expensed as used.
Services Agreement
Neptune provides us with the services of personnel for its administrative, legal and laboratory work as part of a shared service agreement. The employees salaries and benefits are charged proportionally to the time allocation agreed upon. In the three and six-month period ended September 30, 2017, we recognized an expense of $37,000 and $87,000, respectively, in G&A expenses and $8,000 and $20,000, respectively, in R&D expenses under the shared service agreement (for the three and six- month periods ended August 31, 2016$75,000 and $150,000, respectively in G&A expenses, and $9,000 and $9,000, respectively, in R&D expenses).
During the three-month period ended September 30, 2017, the laboratory support, the corporate affairs and the public company reporting services previously provided by Neptune as part of the shared service agreement were discontinued. We are now incurring some incremental costs, and expect to do so in the future, for providing these services directly or through qualified third parties, partially offset by reduced shared service fees. The payable to Neptune primarily for G&A shared services has no specified maturity date for payment or reimbursement and does not bear interest.
Supply of Raw Materials
In the past, Neptune provided us with the krill oil we needed to produce CaPre for our clinical programs. In light of Neptunes recent announcement of its plan to discontinue krill oil production and the sale of its krill oil inventory to Aker, we are evaluating alternative suppliers of krill oil. We believe that alternative supplies of krill oil that can meet our specifications will be readily available.
License Agreement
Under our license agreement with Neptune, we have an exclusive license to use Neptunes intellectual property portfolio related to cardiovascular pharmaceutical applications. The license agreement allows us to develop and
- 100 -
Table of Contents
commercialize CaPre and our novel and active APIs for the prescription drug and medical food markets. Under applicable patent laws, the patents that we have licensed from Neptune under the license agreement are considered prior art to our patents. We entered into the license agreement with Neptune in order to allow us to develop and commercialize CaPre until these Neptune patents expire. As a result of a royalty prepayment transaction we entered into with Neptune on December 4, 2012, we are no longer required to pay any royalties to Neptune under the license agreement during its term for the use of the licensed intellectual property.
Pledge Agreement
On January 7, 2016 Neptune announced the acquisition of Biodroga Nutraceuticals Inc. As part of this transaction, under a pledge agreement, we pledged $2 million of committed funds to partly guarantee the financing for the transaction. Neptune agreed to pay us an annual fee on the committed funds outstanding at an annual rate of 9% during the first six months and 11% for the remaining term of the pledge agreement. On September 20, 2016, Neptune fully released the pledged amount. We recognized interest revenue in the amount of nil for the three and six-month period ended September 30, 2017 and $38,000 and $83,000, respectively, for the three and six-month period ended August 31, 2016.
- 101 -
Table of Contents
PRICE RANGE OF COMMON SHARES AND TRADING MARKETS
Price History
Since March 31, 2011, our common shares have been listed on the TSXV under the ticker symbol APO. Since January 7, 2013, our common shares have been listed on the NASDAQ Stock Market under the ticker symbol ACST. The following tables set forth, for the periods indicated, the high and low market prices of our common shares as reported on the TSXV and the NASDAQ Stock Market.
(a) For the five most recent full fiscal years:
| TSXV | NASDAQ Stock Market | |||||||||||||||
| Fiscal year ended |
High $ | Low $ | High US$ | Low US$ | ||||||||||||
| Feb. 28, 2013(1) |
27.60 | 16.00 | 39.90 | 20.00 | ||||||||||||
| Feb. 28, 2014(1) |
43.20 | 11.50 | 42.00 | 10.90 | ||||||||||||
| Feb. 28, 2015(1) |
14.90 | 11.50 | 13.40 | 10.90 | ||||||||||||
| Feb. 29, 2016 |
7.60 | 1.83 | 6.10 | 1.30 | ||||||||||||
| Mar. 31, 2017 |
4.03 | 1.47 | 3.09 | 1.11 | ||||||||||||
| (1) | Our common shares were consolidated on October 15, 2015, on the basis of one (1) post-consolidation common share for every 10 pre-consolidation common shares, and each fractional common share resulting from the consolidation was rounded up. The common share price was increased proportionally to reflect the consolidation. |
(b) For each full financial quarter of the two most recent full fiscal years and any subsequent period:
| TSXV | NASDAQ Stock Market | |||||||||||||||
| Period |
High $ | Low $ | High US$ | Low US$ | ||||||||||||
| 1st Quarter ended May 31, 2015(1) |
7.60 | 4.00 | 6.10 | 5.00 | ||||||||||||
| 2nd Quarter ended Aug. 31, 2015(1) |
5.50 | 3.50 | 4.20 | 3.90 | ||||||||||||
| 3rd Quarter ended Nov. 30, 2015(1) |
4.70 | 2.65 | 3.80 | 2.01 | ||||||||||||
| 4th Quarter ended Feb. 29, 2016 |
4.40 | 1.83 | 3.20 | 1.30 | ||||||||||||
| 1st Quarter ended May 31, 2016 |
2.45 | 1.50 | 1.88 | 1.20 | ||||||||||||
| 2nd Quarter ended Aug. 31, 2016 |
2.25 | 1.66 | 1.79 | 1.21 | ||||||||||||
| 3rd Quarter ended Nov. 30, 2016 |
4.03 | 1.62 | 3.09 | 1.20 | ||||||||||||
| Four-month period ended Mar. 31, 2017 |
2.66 | 1.47 | 2.03 | 1.11 | ||||||||||||
| 1st Quarter ended June 30, 2017 |
1.96 | 1.65 | 1.51 | 1.23 | ||||||||||||
| 2nd Quarter ended September 30, 2017 |
1.97 | 1.57 | 1.45 | 1.24 | ||||||||||||
| (1) | Our common shares were consolidated on October 15, 2015, on the basis of one (1) post-consolidation common share for every 10 pre-consolidation common shares, and each fractional common share resulting from the consolidation was rounded up. The common share price was increased proportionally to reflect the consolidation. |
(c) For the most recent six months:
| TSXV | NASDAQ Stock Market | |||||||||||||||
| Period |
High $ | Low $ | High US$ | Low US$ | ||||||||||||
| June 2017 |
1.96 | 1.66 | 1.51 | 1.24 | ||||||||||||
| July 2017 |
1.74 | 1.60 | 1.45 | 1.26 | ||||||||||||
| August 2017 |
1.75 | 1.60 | 1.40 | 1.24 | ||||||||||||
| September 2017 |
1.97 | 1.57 | 1.45 | 1.27 | ||||||||||||
| October 2017 |
1.77 | 1.61 | 1.42 | 1.28 | ||||||||||||
| November 2017 |
2.99 | 1.57 | 3.36 | 1.21 | ||||||||||||
- 102 -
Table of Contents
The holders of common shares are entitled to vote at all meetings of our shareholders except meetings at which only holders of a specified class or series of shares are entitled to vote. The holders of common shares are entitled to receive dividends as and when declared by the board, if any.
No common shares have been issued subject to call or assessment. There are no pre-emptive or conversion rights and no provisions for redemption or purchase for cancellation, surrender, or sinking or purchase funds. Our common shares must be issued as fully-paid and non-assessable, and are not subject to further capital calls by us. All of the common shares rank equally as to voting rights, participation in a distribution of our assets on a liquidation, dissolution or winding-up, and the entitlement to dividends. Common shares are transferable at the offices of our transfer agent and registrar, Computershare Trust Company of Canada, in Toronto, Ontario, Canada and Montreal, Québec, Canada. There are no restrictions in our corporate documents on the free transferability of the common shares.
- 103 -
Table of Contents
The Benchmark Company, LLC is acting as book-running manager of this offering and as representative of the underwriters named below. We have entered into an underwriting agreement with the underwriters with respect to the common shares and warrants being offered. Subject to the terms and conditions of the underwriting agreement, we have agreed to sell to the underwriters, and each underwriter has severally agreed to purchase, at the public offering price less the underwriting discounts and commissions set forth on the cover page of this prospectus, the number of common shares and warrants listed next to its name in the following table:
| Name |
Number of common shares |
Number of warrants |
||||||
| The Benchmark Company, LLC |
4,950,495 | 4,455,446 | ||||||
| Dawson James Securities, Inc. |
4,950,495 | 4,455,445 | ||||||
|
|
|
|
|
|||||
| Total |
9,900,990 | 8,910,891 | ||||||
|
|
|
|
|
|||||
The underwriters are committed to purchase all the common shares and warrants offered by us if they purchase any such securities. The underwriting agreement also provides that if an underwriter defaults, the purchase commitments of non-defaulting underwriters may also be increased or the offering may be terminated.
The underwriters propose to offer the common shares and warrants directly to the public at the public offering price set forth on the cover page of this prospectus and to certain dealers at that price per share and accompanying warrant less a concession not in excess of US$0.0505 per share and accompanying warrant.
We have granted the underwriters an option to buy from us up to an additional 1,100,110 common shares and/or warrants to purchase up to an aggregate of 990,099 common shares at an exercise price of US$1.26 per share, in any combination thereof to cover over-allotments. The underwriters have 30 days from the date of this prospectus to exercise this option to purchase additional common shares and warrants. If any additional common shares are purchased, the underwriters will offer the additional common shares on the same terms as those on which the common shares are being offered.
The underwriting fee is equal to the public offering price per share and accompanying warrant less the amount paid by the underwriters to us per share and accompanying warrant. The underwriting fee is US$0.0707 per common share and accompanying warrant. The following table shows the per share and total underwriting discounts and commissions to be paid to the underwriters assuming both no exercise and full exercise of the underwriters option to purchase additional shares and warrants.
| No exercise of option |
Full exercise of option |
|||||||
| Per share and warrant |
US$ | 0.0707 | US$ | 0.0707 | ||||
|
|
|
|
|
|||||
| Total |
US$ | 700,000 | US$ | 777,778 | ||||
|
|
|
|
|
|||||
We have also agreed to reimburse the underwriters for their expenses in connection with this offering, up to US$125,000. We estimate that the total expenses of this offering, including registration, filing and listing fees, printing fees and legal and accounting expenses, but excluding the underwriting discounts and commissions, will be approximately US$687,000.
We have also agreed to issue to the underwriters a warrant to purchase a number of our common shares equal to an aggregate of 5% of the common shares sold in this offering. The underwriters warrant will have an exercise price equal to 125% of the public offering price of the shares set forth on the cover of this prospectus (or US$1.26 per share) and may be exercised on a cashless basis. The underwriters warrant is not redeemable by us. The underwriters warrants provide for piggy-back registration rights for a period of five years. The underwriters warrant and the underlying common shares have been deemed compensation by FINRA, and are therefore subject to FINRA Rule 5110(g)(1). In accordance with FINRA Rule 5110(g)(1), neither the
- 104 -
Table of Contents
underwriters warrant nor any common shares issued upon exercise of the underwriters warrant may be sold, transferred, assigned, pledged, or hypothecated, or be the subject of any hedging, short sale, derivative, put, or call transaction that would result in the effective economic disposition of such securities by any person for a period of 180 days immediately following the date of effectiveness or commencement of sales of the offering pursuant to which the underwriters warrant is being issued, except the transfer of any security: (i) by operation of law or by reason of reorganization of our company; (ii) to any FINRA member firm participating in this offering and the officers or partners thereof, if all securities so transferred remain subject to the lock-up restriction described above for the remainder of the time period; (iii) if the aggregate amount of our securities held by either an underwriter or a related person do not exceed 1% of the securities being offered; (iv) that is beneficially owned on a pro-rata basis by all equity owners of an investment fund, provided that no participating member manages or otherwise directs investments by the fund, and participating members in the aggregate do not own more than 10% of the equity in the fund; or (v) the exercise or conversion of any security, if all securities received remain subject to the lock-up restriction set forth above for the remainder of the time period. In addition, in accordance with FINRA Rule 5110(f)(2)(G), the underwriters warrant may not contain certain anti-dilution terms.
We, our officers and directors and certain of our shareholders have agreed to a six-month lock up with respect to our common shares and other of our securities beneficially owned, including securities that are convertible into, or exchangeable or exercisable for, common shares. This means that, subject to certain exceptions, for a period of six months following the date of this prospectus, we and such persons may not offer, sell, pledge or otherwise dispose of any such securities without the prior written consent of the underwriters. Notwithstanding the forgoing, private transactions in our securities may occur provided the transferee agrees to be bound by the same terms as set forth above.
We have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act.
In connection with this offering, the underwriters may engage in stabilizing transactions, which involves making bids for, purchasing and selling common shares in the open market for the purpose of preventing or retarding a decline in the market price of the common shares while this offering is in progress. These stabilizing transactions may include making short sales of the common shares, which involves the sale by the underwriters of a greater number of common shares than they are required to purchase in this offering, and purchasing common shares on the open market to cover positions created by short sales. Short sales may be covered shorts, which are short positions in an amount not greater than the underwriters option to purchase additional shares referred to above, or may be naked shorts, which are short positions in excess of that amount. The underwriters may close out any covered short position either by exercising their option to purchase additional shares, in whole or in part, or by purchasing shares in the open market. In making this determination, the underwriters will consider, among other things, the price of shares available for purchase in the open market compared to the price at which the underwriters may purchase shares through the option to purchase additional shares. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the common shares in the open market that could adversely affect investors who purchase in this offering. To the extent that the underwriters create a naked short position, they will purchase shares in the open market to cover the position.
These activities may have the effect of raising or maintaining the market price of the common shares or preventing or retarding a decline in the market price of the common shares, and, as a result, the price of the common shares may be higher than the price that otherwise might exist in the open market. If the underwriters commence these activities, they may discontinue them at any time. The underwriters may carry out these transactions on the NASDAQ Stock Market, in the over-the-counter market or otherwise.
In addition, in connection with this offering certain of the underwriters (and selling group members) may engage in passive market making transactions in our common shares on the NASDAQ Stock Market prior to the pricing and completion of this offering. Passive market making consists of displaying bids on the NASDAQ Stock Market no higher than the bid prices of independent market makers and making purchases at prices no higher
- 105 -
Table of Contents
than these independent bids and effected in response to order flow. Net purchases by a passive market maker on each day are generally limited to a specified percentage of the passive market makers average daily trading volume in the common shares during a specified period and must be discontinued when such limit is reached. Passive market making may cause the price of our common shares to be higher than the price that otherwise would exist in the open market in the absence of these transactions. If passive market making is commenced, it may be discontinued at any time.
The underwriters or their affiliates may engage in transactions with, and may perform, from time to time, investment banking and advisory services for us in the ordinary course of their business and for which they would receive customary fees and expenses. However, except as disclosed in this prospectus, we have no present arrangements with any of the underwriters for any further services.
We expect that delivery of the common shares and accompanying warrants will be made to investors on or about December 27, 2017, which will be the third business day following the date of this prospectus (such settlement being referred to as T+3). Under Rule 15c6-1 under the Securities Exchange Act of 1934, trades in the secondary market are required to settle in two business days, unless the parties to any such trade expressly agree otherwise. Accordingly, purchasers who wish to trade such securities on any date prior to two business days before delivery will be required, by virtue of the fact that the securities initially settle in T+3, to specify an alternate settlement arrangement at the time of any such trade to prevent a failed settlement. Purchasers of the common shares and accompanying warrants who wish to trade the securities on any date prior to two business days before delivery should consult their advisors.
International Selling Restrictions
Other than in the United States, no action has been taken by us or the underwriters that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
- 106 -
Table of Contents
EXPENSES RELATING TO THIS OFFERING
The following table sets forth the estimated costs and expenses, other than underwriting discounts and commissions, payable by us in connection with this offering.
| SEC registration fee |
US$ | 4,700 | ||
| Printing expenses |
US$ | 25,000 | ||
| Accounting fees and expenses |
US$ | 125,000 | ||
| Legal fees and expenses |
US$ | 530,000 | ||
| Financial Industry Regulatory Authority, Inc. filing fee |
US$ | 3,000 | ||
|
|
|
|||
| Total |
US$ | 687,700 | ||
|
|
|
- 107 -
Table of Contents
Overview
Our authorized capital consists of an unlimited number of no par value common shares and an unlimited number of no par value Class B, Class C, Class D and Class E preferred shares (collectively, the preferred shares), issuable in one or more series. As of December 15, 2017, there were:
| | a total of 14,914,658 common shares issued and outstanding and no preferred shares issued and outstanding; |
| | 2,395,788 options to purchase common shares issued to our directors, officers and employees, at a weighted average exercise price of $1.82 per common share; |
| | 18,400,000 Series 8 public offering warrants issued in 2014 to purchase common shares issued and outstanding (including 592,500 warrants held by Neptune), exercisable at a price of US$15.00 per common share until December 3, 2018 (10 warrants must be exercised in order to acquire one common share); |
| | 161,654 Series 9 private placement warrants issued in 2014 to purchase common shares issued and outstanding, exercisable at a price of $13.30 per common share until December 3, 2018; |
| | $2,000,000 aggregate principal amount of unsecured convertible debentures, maturing on February 21, 2020, issued in our February 2017 private placement and contingent warrants to acquire up to 1,052,630 common shares: |
| | the debentures are convertible into common shares at any time by the holder at a fixed price of $1.90 per common share, except if we pay before the maturity all or any portion of the convertible debentures; |
| | the contingent warrants will be exercisable for the remaining term of the convertible debentures at a fixed price of $1.90 per common share; |
| | warrants issued in connection with our February 2017 public offering to purchase up to 1,904,034 common shares at an exercise price of $2.15 per common share, at any time until February 21, 2022; and |
| | broker warrants issued in connection with our February 2017 public offering to purchase up to 117,496 common shares at an exercise price of $2.15 per common share, at any time until February 21, 2018. |
The following is a brief description of the rights, privileges, conditions and restrictions attaching to the common shares and preferred shares.
Common Shares
Voting Rights
Each common share entitles its holder to receive notice of, and to attend and vote at, all annual or special meetings of our shareholders. Each common share entitles its holder to one vote at any meeting of our shareholders, other than meetings at which only the holders of a particular class or series of shares are entitled to vote due to statutory provisions or the specific attributes of this class or series.
Dividends
Subject to the prior rights of the holders of preferred shares ranking before the common shares as to dividends, the holders of common shares are entitled to receive dividends as declared by the board our funds that are available for the payment of dividends.
- 108 -
Table of Contents
Winding-up and Dissolution
In the event of our voluntary or involuntary winding-up or dissolution, or any other distribution of our assets among our shareholders for the purposes of winding up its affairs, the holders of common shares shall be entitled to receive, after payment by us to the holders of preferred shares ranking prior to common shares regarding the distribution of our assets in the case of winding-up or dissolution, share for share, the remainder of our property, with neither preference nor distinction. The order of priority, applicable to all classes of our shares with respect to the redemption, liquidation, dissolution or distribution of property (the order of priority) is as follows: First, the Class E non-voting shares; Second, the Class D non-voting shares; Third, the Class B multiple voting shares and Class C non-voting shares, pari passu; and Fourth, the common shares. Notwithstanding the order of priority, shareholders of a class of shares may renounce the order of priority by unanimous approval by all shareholders of that class of shares.
Preferred Shares
Class B Multiple Voting Shares
Each Class B multiple voting share entitles the holder thereof to 10 votes per share in all of our shareholder meetings.
Dividends. Holders of Class B multiple voting shares are entitled to receive, as and when such dividends are declared, an annual non-cumulative dividend of 5% on the amount paid for the said shares, payable at the time and in the manner which the directors may determine and subject to the order of priority.
Participation. Subject to the provisions of subsection 5.2.2 of our Articles, holders of Class B multiple voting shares do not have the right to participate in our profits or surplus assets.
Conversion. Holders of Class B multiple voting shares have the right, at their entire discretion, to convert, part or all of the Class B multiple voting shares they hold into common shares on the basis of 1 common share for each Class B multiple voting share converted.
Redemption. Subject to the provisions of the QBCA and the order of priority, holders of Class B multiple voting shares have the right to demand from us, upon 30 days written notice, that we redeem the Class B multiple voting shares at a price equivalent to the amount paid for such shares plus the redemption premium, as defined in subsection 5.2.4.1 of the Articles, and any and all declared but yet unpaid dividends on same.
Liquidation. In the event of our dissolution or liquidation or any other distribution of our property, the Class B voting shareholders have the right to be reimbursed for the amount paid for their Class B multiple voting shares plus the redemption premium, as defined in subsection 5.2.4.1 of our Articles as well as the amount of any and all declared but yet unpaid dividends on their shares, subject to the order of priority.
Class C Non-Voting Shares
Subject to the provisions of the QBCA, holders of Class C non-voting shares are neither entitled to vote at any meeting of our shareholders, receive a notice of any such meeting, nor attend any such meeting.
Dividends. Holders of Class C non-voting shares are entitled to receive, as and when such dividends are declared, an annual non-cumulative dividend of 5% on the amount paid for the said shares, plus a redemption premium as defined in subsection 5.3.6.1 of our Articles, payable at the time and in the manner which the directors may determine and subject to the order of priority.
Participation. Subject to the provisions of subsection 5.3.2 of our Articles, holders of Class C non-voting shares do not have the right to participate in our profits or surplus assets.
- 109 -
Table of Contents
Conversion. Holders of Class C non-voting shares have the right, at their entire discretion, to convert, part or all of the Class C non-voting shares they hold into common shares on the basis of 1 common share for each Class C non-voting share converted.
Forced Conversion. All of our Class C non-voting shares shall automatically be converted in common shares upon the request of an unrelated third-party investor in us investing more than $500,000, or any other amount to be determined by the board of directors in us and requesting as a condition to the investment that the Class C non-voting shares be converted into common shares on the basis of 1 common share for each Class C non-voting share converted.
Redemption. Subject to the provisions of the QBCA and the order of priority, holders of Class C non-voting shares have the right to demand, upon 30 days written notice, that we redeem their Class C non-voting shares at a price equivalent to the amount paid for the shares plus the redemption premium, as defined in subsection 5.3.6.1 of our Articles, and any and all declared but yet unpaid dividends on the shares.
Liquidation. In the event of our dissolution or liquidation or any other distribution of our property, Class C non-voting shareholders have the right to be reimbursed for the amount paid for their Class C non-voting shares plus the redemption premium, as defined in subsection 5.3.6.1 of our Articles, as well as the amount of any and all declared but yet unpaid dividends on their shares, subject to the order of priority.
Class D Non-Voting Shares
Subject to the provisions of the QBCA, holders of Class D non-voting shares are neither entitled to vote at any meeting of the shareholders, receive a notice of any such meeting, nor attend any such meeting.
Dividends. Holders of Class D non-voting shares are entitled to receive, as and when such dividends are declared, a monthly non-cumulative dividend of 0.5% to 2% on the amount paid for the shares, plus a redemption premium as defined in subsection 5.4.6.1 of our Articles, payable at the time and in the manner which the directors may determine and subject to the order of priority.
Participation. Subject to the provisions of subsection 5.4.2 of our Articles, holders of Class D non-voting shares do not have the right to participate in our profits or surplus assets.
Conversion. Holders of Class D non-voting shares have the right, at their discretion, to convert, part or all of their Class D non-voting shares into common shares on the basis of a number of common shares equal to the number of Class D non-voting shares converted multiplied by a conversion ratio, calculated as follows:
| Conversion Ratio = |
The product obtained by multiplying a factor to be agreed at the time of the issuance of the Class D non-voting shares by the average amount paid per share for the Class D non-voting shares plus the redemption premium per share, as defined in subsection 5.4.6.1 of our Articles as well as the amount of any and all declared but yet paid dividends on the shares |
|
| Fair market value of the common shares at the date of any conversion of Class D non-voting shares into common shares |
- 110 -
Table of Contents
Conversion All of our Class C non-voting shares automatically convert into common shares upon the request of an unrelated third party investor in us, investing more than $500,000, or any other amount to be determined by the board of directors, in us and requesting as a condition to the investment that the Class C non-voting shares be converted into common shares in all cases, on the basis of a number of common shares equal to the number of Class D non-voting shares converted multiplied by the conversion ratio, calculated as follows:
| Conversion Ratio = |
The product obtained by multiplying a factor to be agreed at the time of the issuance of the Class D non-voting shares by the average amount paid per share for the Class D non-voting shares plus the redemption premium per share, as defined in subsection 5.4.6.1 of our Articles as well as the amount of any and all declared but yet paid dividends on the shares |
|
| Fair market value of the common shares at the date of any conversion of Class D non-voting shares into common shares |
Redemption. Subject to the provisions of the QBCA and the order of priority, holders of Class D non-voting shares have the right to demand, upon 30 days written notice, that we redeem their Class D non-voting shares at a price equivalent to the amount paid for the shares plus the redemption premium, as defined in subsection 5.4.6.1 of our Articles, and any and all declared but yet unpaid dividends on the shares.
Liquidation. In the event of our dissolution or liquidation or any other distribution of our property, the Class D non-voting shareholders shall have the right to be reimbursed for the amount paid for their Class D non-voting shares plus the redemption premium, as defined in subsection 5.4.6.1 of our Articles as well as the amount of any and all declared but yet unpaid dividends on their shares, subject to the order of priority.
Class E Non-Voting Shares
Subject to the provisions of the QBCA, holders of Class E non-voting shares are neither entitled to vote at any meeting of the shareholders, receive a notice of any such meeting, nor attend any such meeting.
Dividends. Holders of Class E non-voting shares are entitled to receive, as and when such dividends are declared, a monthly non-cumulative dividend of 0.5% to 2% on the amount paid for the shares, payable at the time and in the manner which the directors may determine and subject to the order of priority.
Participation. Subject to the provisions of subsection 5.5.2 of our Articles, holders of Class E non-voting shares do not have the right to participate in our profits.
Conversion. Holders of Class E non-voting shares have the right, at their discretion, to convert, part or all of their Class E non-voting shares into common shares on the basis of a number of common shares equal to the number of Class E non-voting shares converted multiplied by the conversion ratio, calculated as follows:
| Conversion Ratio = |
The product obtained by multiplying a factor to be agreed at the time of the issuance of the Class E non-voting shares by the average amount paid per share for the Class E non-voting shares plus the amount of any and all declared but yet paid dividends on the shares |
|
| Fair market value of the common shares at the date of any conversion of Class E non-voting shares into common shares |
Redemption. Subject to the provisions of the QBCA and the order of priority, we have the right, upon 30 days written notice, to redeem the Class E non-voting shares at a price equivalent to the amount paid for the shares and any and all declared but yet unpaid dividends on the shares.
Liquidation. In the event of our dissolution or liquidation or any other distribution of our property, the Class E non-voting shareholders have the right to be reimbursed for the amount paid for their Class E non-voting shares as well as the amount of any and all declared but yet unpaid dividends on the shares, subject to the order of priority.
- 111 -
Table of Contents
Warrants
The warrants to be issued in this offering entitle the registered holder to purchase one common share at a price of US$1.26 per share, subject to adjustment as discussed below, immediately following the issuance of such warrant and terminating at 5:00 p.m., New York City time, five years after the closing of this offering.
The warrants will be issued pursuant to a warrant agreement between us and the warrant agent. Certain provisions of the warrants are set forth herein but are only a summary and are qualified in their entirety by the relevant provisions of the warrant agreement, the form of which is filed as an exhibit to the registration statement of which this prospectus forms a part. The exercise price and number of common shares issuable upon exercise of the warrants may be adjusted in certain circumstances, including in the event of a share dividend or recapitalization, reorganization, merger or consolidation. However, the warrants will not be adjusted for issuances of common shares at prices below their exercise price.
The warrants may be exercised upon surrender of the warrant certificate on or prior to the expiration date at the offices of the warrant agent, with the exercise form on the reverse side of the warrant certificate completed and executed as indicated, accompanied by full payment of the exercise price, by certified or official bank check payable to us, for the number of warrants being exercised. Holders of the warrants will have the right to exercise the warrants via a cashless exercise feature provided for in the warrants if at the time of exercise of the warrants there is not an effective registration statement and current prospectus for the issuance of the underlying common shares. The warrant holders do not have the rights or privileges of holders of common shares or any voting rights until they exercise their warrants and receive the common shares. After the issuance of common shares upon exercise of the warrants, each holder will be entitled to one vote for each share held of record on all matters to be voted on by shareholders.
A holder may not exercise any portion of a warrant to the extent that the holder, together with its affiliates and any other person or entity acting as a group, would own more than 4.99% of our outstanding common shares after exercise, as such percentage ownership is determined in accordance with the terms of the warrant, except that upon at least 61 days prior notice from the holder to us, the holder may waive such limitation up to a percentage not in excess of 9.99%. No fractional common shares will be issued upon exercise of the warrants. If, upon exercise of the warrant, a holder would be entitled to receive a fractional interest in a share, we will, upon exercise, either pay a cash adjustment in respect of such fraction in an amount equal to such fraction multiplied by the exercise price or round up to the next whole share. If multiple warrants are exercised by the holder at the same time, we will pay a cash adjustment in respect of such final fraction in an amount equal to such fraction multiplied by the exercise price or round up to the next whole share.
- 112 -
Table of Contents
MEMORANDUM AND ARTICLES OF INCORPORATION
Memorandum and Articles of Association
We were incorporated on February 1, 2002 under Part 1A of the Companies Act (Québec) under the name 9113-0310 Québec Inc. On August 7, 2008, pursuant to a Certificate of Amendment, we changed our name to Acasti Pharma Inc., our share capital, the provisions regarding the restrictions on securities transfers and our borrowing powers. On November 7, 2008, pursuant to a Certificate of Amendment, we further revised our provisions regarding our borrowing powers. We became a reporting issuer in Québec on November 17, 2008. On February 14, 2011, the Business Corporations Act (Québec) came into effect and replaced the Companies Act (Québec). We are now governed by the Business Corporations Act (Québec), or the QBCA.
Register, Entry Number and Purposes
Our articles of incorporation, as amended, or Articles, and general by-laws, do not define any of our objects and purposes. In that respect, we have no limit on the type of business we can carry out.
Directors Powers
Our Articles and by-laws do not contain any provision regarding: (a) a directors power in the absence of an independent quorum, to vote compensation to itself or any members of the committees of the board; (b) retirement or non-retirement of directors under an age limit requirement; and (c) number of shares, if any, required for a directors qualification.
Our by-laws provide that a director may not vote on a resolution to approve, amend or terminate a contract or transaction in which the director has any financial stake that may reasonably be considered to influence decision-making or be present during deliberations concerning the approval, amendment or termination of such a contract or transaction, unless the contract or transaction: (a) relates primarily to the remuneration of the director or an associate of the director as a director of us or an affiliate of us, (b) relates primarily to the remuneration of the director or an associate of the director as an officer, employee or mandatary of us or an affiliate of us, if we are not a reporting issuer, (c) is for indemnity or liability insurance, or (d) is with an affiliate of us, and the sole interest of the director is as a director or officer of the affiliate. In addition, our by-laws provide that a director must avoid placing himself or herself in any situation where his or her personal interests would be in conflict with his obligations as a director of ours, and that a director must disclose to us any interest he or she has in a business or association that may place him or her in a situation of conflict of interest and of any right he or she may set up against us, indicating their nature and value, where applicable.
Our Articles provide that the board may, on behalf us, (a) borrow money, (b) issue, reissue, sell or pledge debt instruments, (c) guarantee the obligations of a third party, and (d) hypothecate all or any of its assets, both present and future, to guarantee the performance of any of our obligations.
The quorum at every meeting of the board has been set to the minimum number of directors required under our Articles. In the absence of a quorum, a director has no power to make any decision regarding, among other things, compensation to himself or herself or to any member of the committees of the board.
Our by-laws do not contain any requirements with respect to a mandatory retirement age for our directors and the number of shares required for directors qualifications.
Procedures to Change the Rights of Shareholders
In order to change the rights attached to all classes of our shares, the vote of at least 66 2/3% of the holders of each class, must be cast at a shareholders meeting called for amending the rights attached to our common shares or preferred shares, as the case may be.
- 113 -
Table of Contents
Ordinary and Extraordinary Shareholders Meetings
Our by-laws provide that our annual meeting of shareholders must be held on a yearly basis on such date and on such time as may be fixed by the board. Our by-laws provide that special meetings of shareholders may be called at any time as determined by the board. Our shareholders are entitled to call special meetings of shareholders, provided that they hold at least 10% of the issued and outstanding shares entitled to vote at the meeting so called. Our by-laws provide that notice of each annual and special meeting of shareholders must be sent to the shareholders entitled to attend such meetings not less than 21 days and not more than 60 days before the date fixed for such meeting. Our by-laws provide that during any meeting of shareholders, the attendance, in person or by proxy, of at least two shareholders representing at least 10% of the issued and outstanding shares entitled to vote at the meeting will constitute a quorum.
Our bylaws require that advance notice be given to us in circumstances where nominations of persons for election as a director are made by shareholders other than pursuant to a requisition of a meeting made pursuant to the provisions of the QBCA or a shareholder proposal made pursuant to the provisions of the QBCA.
Among other things, the advance notice bylaw fixes a deadline by which shareholders must submit a notice of director nominations to us prior to any annual or special meeting of shareholders where directors are to be elected and sets forth the information that a shareholder must include in the notice for it to be valid.
In the case of an annual meeting of shareholders, notice must be given to us no less than 30 nor more than 65 days prior to the date of the annual meeting provided, however, that in the event that the annual meeting is to be held on a date that is less than 50 days after the date on which the first public announcement of the date of the annual meeting was made, notice may be given no later than the close of business on the 10th day following such public announcement. In the case of a special meeting of shareholders (which is not also an annual meeting), notice must be given to us no later than the close of business on the 15th day following the day on which the first public announcement of the date of the special meeting was made.
Limitations on Rights to Own Securities
There exists no limitation on the right to own our securities.
Impediments to Change of Control
Neither our Articles nor by-laws contain any provision that would have an effect of delaying, deferring or preventing a change in control of us.
Stockholder Ownership Disclosure Threshold in Bylaws
Our Articles and By-laws do not contain any provision requiring a shareholder to disclose his ownership above a particular threshold.
- 114 -
Table of Contents
Differences in Corporate Law
We are governed by the QBCA which is generally similar to laws applicable to United States corporations. Significant differences between the QBCA and the Delaware General Corporation Law, or DGCL, which governs companies incorporated in the State of Delaware, include the differences summarized below. This summary is not an exhaustive review of the two statutes, and reference should be made to the full text of both statutes for particulars of the differences.
| Number and Election of Directors |
||
| Delaware |
Quebec | |
| Under the DGCL, the board of directors must consist of at least one number. The number of directors shall be fixed by the bylaws of the corporation, unless the certificate of incorporation fixes the number of directors, in which case a change in the number of directors shall only be made by an amendment of the certificate of incorporation. Under the DGCL, directors are elected at annual stockholder meetings by plurality vote of the stockholders, unless a shareholder-adopted bylaw prescribes a different required vote. |
Under the QBCA, the board of directors of a corporation must consist of at least three members, at least two of whom must not be officers or employees of the corporation or an affiliate of the corporation, so long as the corporation remains a reporting issuer for purposes of the QBCA, which includes a corporation that has made a distribution of securities to the public. Under the QBCA, directors are elected by the shareholders, in the manner and for the term, not exceeding three years, set out in the corporations bylaws. Our bylaws provide that our directors are elected at each annual meeting of shareholders at which such an election is required. |
|
| Removal of Directors |
||
| Delaware |
Quebec | |
| Under the DGCL, any or all directors may be removed with or without cause by the holders of a majority of shares entitled to vote at an election of directors unless the certificate of incorporation otherwise provides or in certain other circumstances if the corporation has cumulative voting. |
Under the QBCA, unless the articles of a corporation provide for cumulative voting (which is not the case for us), shareholders of the corporation may, by resolution passed by a majority of the vote cast thereon at a special meeting of shareholders, remove any or all directors from office and may elect any qualified person to fill the resulting vacancy. |
|
| Vacancies on the Board of Directors |
||
| Delaware |
Quebec | |
| Under the DGCL, vacancies and newly created directorships resulting from an increase in the authorized number of directors, may be filled by a majority of the directors then in office, although less than a quorum, or by a sole remaining director. |
Under the QBCA, vacancies that exist on the board of directors may generally be filled by the board if the remaining directors constitute a quorum. In the absence of a quorum, the remaining directors shall call a meeting of shareholders to fill the vacancy. |
|
| Board of Director Quorum and Vote Requirements |
||
| Delaware |
Quebec | |
| Under the DGCL, a majority of the total number of directors shall constitute a quorum for the transaction of |
Under the QBCA, subject to the corporations bylaws, a majority of the directors in office |
|
- 115 -
Table of Contents
| business unless the certificate or bylaws require a greater number. The bylaws may lower the number required for a quorum to one-third the number of directors, but no less.
Under the DGCL, the board of directors may take action by the majority vote of the directors present at a meeting at which a quorum is present unless the certificate of incorporation or bylaws require a greater vote. |
constitutes a quorum at any meeting of the board. Our bylaws also provide that a majority of the directors in office constitutes a quorum at any meeting of the board.
Under the QBCA, a quorum of directors may exercise all the powers of the directors despite any vacancy on the board. |
|
| Transactions with Directors and Officers |
||
| Delaware |
Quebec | |
| The DGCL generally provides that no transaction between a corporation and one or more of its directors or officers, or between a corporation and any other corporation or other organization in which one or more of its directors or officers, are directors or officers, or have a financial interest, shall be void or voidable solely for this reason, or solely because the director or officer is present at or participates in the meeting of the board or committee which authorizes the transaction, or solely because any such directors or officers votes are counted for such purpose, if (i) the material facts as to the directors or officers interest and as to the transaction are known to the board of directors or the committee, and the board or committee in good faith authorizes the transaction by the affirmative votes of a majority of the disinterested directors, even though the disinterested directors be less than a quorum (ii) the material facts as to the directors or officers interest and as to the transaction are disclosed or are known to the stockholders entitled to vote thereon, and the transaction is specifically approved in good faith by vote of the stockholders; or (iii) the transaction is fair as to the corporation as of the time it is authorized, approved or ratified, by the board of directors, a committee or the stockholders. |
Under the QBCA, every director or officer of a corporation must disclose the nature and value of any interest he or she has in a contract or transaction to which the corporation is a party. For the purposes of this rule, interest means any financial stake in a contract or transaction that may reasonably be considered likely to influence decision-making. Furthermore, a proposed contract or a proposed transaction, including related negotiations, is considered a contract or transaction. In addition, a director or an officer must disclose any contract or transaction to which the corporation and any of the following are a party: (i) an associate of the director or officer; (ii) a group of which the director or officer is a director or officer; or (iii) a group in which the director or officer or an associate of the director or officer has an interest. Such disclosure is required even for a contract or transaction that does not require approval by the board of directors. If a director is required to disclose his or her interest in a contract or transaction, such director is not allowed to vote on any resolution to approve, amend or terminate the contract or transaction or be present during deliberations concerning the approval, amendment or termination of such contract or transaction, unless the contract or transaction (i) relates primarily to the remuneration of the director or an associate of the director as a director, officer, employee or mandatory of the corporation or an affiliate of the corporation, (ii) is for indemnity or liability insurance under the QBCA, or (iii) is with an affiliate of the corporation, and the sole interest of the director is as a director or officer of the affiliate.
If a director or officer does not disclose his or her interest in accordance with the QBCA, or (in the case of a director) votes in respect of a resolution on a contract or transaction in which he or she is |
|
- 116 -
Table of Contents
| interested contrary to the QBCA, the corporation or a shareholder may ask the court to declare the contract or transaction null and to require the director or officer to account to the corporation for any profit or gain realized on it by the director or officer or the associates of the director or officer, and to remit the profit or gain to the corporation, according to the conditions the court considers appropriate. However, the contract or transaction may not be declared null if it was approved by the board of directors and the contract or transaction was in the interest of the corporation when it was approved, nor may the director or officer concerned, in such a case, be required to account for any profit or gain realized or to remit the profit or gain to the corporation. In addition, the contract or transaction may not be declared null if it was approved by ordinary resolution by the shareholders entitled to vote who do not have an interest in the contract or transaction, the required disclosure was made to the shareholders and the contract or transaction was in the best interests of the corporation when it was approved, and if the director or officer acted honestly and in good faith, he or she may not be required to account for the profit or gain realized and to remit the profit or gain to the corporation. |
||
| Limitation on Liability of Directors |
||
| Delaware |
Quebec | |
| The DGCL permits a corporation to include a provision in its certificate of incorporation eliminating or limiting the personal liability of a director to the corporation or its stockholders for monetary damages for a breach of the directors fiduciary duty as a director, except for liability:
for breach of the directors duty of loyalty to the corporation or its stockholders;
for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of the law;
under Section 174 of the DGCL, which concerns unlawful payment of dividends, stock purchases or redemptions; or
for any transaction from which the director derived an improper personal benefit |
The QBCA does not permit the limitation of a directors liability as the DGCL does. |
|
- 117 -
Table of Contents
| Indemnification of Directors and Officers |
||
| Delaware |
Quebec | |
| The DGCL permits indemnification for derivative suits only for expenses (including legal fees) and only if the person is not found liable, unless a court determines the person is fairly and reasonably entitled to the indemnification. |
Under the QBCA, a corporation may indemnify a director or officer, a former director or officer or a person who acts or acted at the corporations request as a director or officer, or an individual acting in a similar capacity of another group (who is referred to in this document as an indemnifiable person) against all costs, charges and expenses, including an amount paid to settle an action or satisfy a judgment, reasonably incurred by the indemnifiable person on the exercise of the persons functions or arising from any investigative or other proceeding in which the person is involved if:
the person acted honestly and loyalty in the interest of the corporation or other group, and
in the case of a proceeding enforceable by a monetary penalty, the person had reasonable grounds for believing the persons conduct was lawful.
An indemnifiable person is also entitled to indemnity for reasonable defense costs and expenses if the person fulfills the above-mentioned requirements and was not judged to have committed any fault or omitted to do anything the person ought to have done. In the case of a derivative action, indemnity may be made only with court approval. |
|
| Call and Notice of Stockholder Meetings |
||
| Delaware |
Quebec | |
| Under the DGCL, an annual or special stockholder meeting is held on such date, at such time and at such place as may be designated by the board of directors or any other person authorized to call such meeting under the corporations certificate of incorporation or bylaws. If an annual meeting for election of directors is not held on the date designated or an action by written consent to elect directors in lieu of an annual meeting has not been taken within 30 days after the date designated for the annual meeting, or if no date has been designated, for a period of 13 months after the later of the last annual meeting or the last action by written consent to elect directors in lieu of an annual meeting, the Delaware Court of Chancery may summarily order a meeting to be held upon the application of any stockholder or director. |
Under the QBCA, an annual meeting of shareholders must be held no later than fifteen months after holding the last preceding annual meeting. Under the QBCA, the directors of a corporation may call a special meeting at any time. In addition, holders of not less than 10 percent of the issued shares of a corporation that carry the right to vote at a meeting sought to be held may requisition the directors to call a meeting of shareholders. |
|
- 118 -
Table of Contents
| Stockholder Action by Written Consent |
||
| Delaware |
Quebec | |
| Under the DGCL, a majority of the stockholders of a corporation may act by written consent without a meeting unless such action is prohibited by the corporations certificate of incorporation. |
Under the QBCA, a written resolution signed by all the shareholders of a corporation who would have been entitled to vote on the resolution at a meeting is effective to approve the resolution. |
|
| Stockholder Nominations and Proposals |
||
| Delaware |
Quebec | |
| Not applicable. |
Under the QBCA, a shareholder entitled to vote at a shareholders meeting may submit a shareholder proposal relating to matters which the shareholder wishes to propose and discuss at an annual shareholders meeting and, subject to such shareholders compliance with the prescribed time periods and other requirements of the QBCA pertaining to shareholder proposals, the corporation is required to include such proposal in the information circular pertaining to any annual meeting at which it solicits proxies, subject to certain exceptions. Notice of such a proposal must be provided to the corporation at least 90 days before the anniversary date of the notice of meeting for the last annual shareholders meeting.
In addition, the QBCA requires that any shareholder proposal that includes nominations for the election of directors must be signed by one or more holders of shares representing in the aggregate not less than five per cent of the shares or five per cent of the shares of a class or series of shares of the corporation entitled to vote at the meeting to which the proposal is to be presented. |
|
| Stockholder Quorum and Vote Requirements |
||
| Delaware |
Quebec | |
| Under the DGCL, quorum for a stock corporation is a majority of the shares entitled to vote at the meeting unless the certificate of incorporation or bylaws specify a different quorum, but in no event may a quorum be less than one-third of the shares entitled to vote. Unless the DGCL, certificate of incorporation or bylaws provide for a greater vote, generally the required vote under the DGCL is a majority of the shares present in person or represented by proxy, except for the election of directors which requires a plurality of the votes cast. |
Under the QBCA, unless the bylaws otherwise provide, the holders of a majority of the shares of a corporation entitled to vote at a meeting of shareholders, whether present in person or represented by proxy, constitute a quorum. |
|
- 119 -
Table of Contents
| Amendment of Governing Instrument |
||
| Delaware |
Quebec | |
| Amendment of Certificate of Incorporation. Generally, under the DGCL, the affirmative vote of the holders of a majority of the outstanding stock entitled to vote is required to approve a proposed amendment to the certificate of incorporation, following the adoption of the amendment by the board of directors of the corporation, provided that the certificate of incorporation may provide for a greater vote. Under the DGCL, holders of outstanding shares of a class or series are entitled to vote separately on an amendment to the certificate of incorporation if the amendment would have certain consequences, including changes that adversely affect the rights and preferences of such class or series. |
Amendment of Articles. Under the QBCA, amendments to the articles of incorporation generally require the approval of not less than two-thirds of the votes cast by shareholders entitled to vote on the resolution. Specified amendments may also require the approval of other classes of shares. If the amendment is of a nature affecting a particular class or series in a manner requiring a separate class or series vote, that class or series is entitled to vote on the amendment whether or not it otherwise carries the right to vote. |
|
| Amendment of Bylaws. Under the DGCL, after a corporation has received any payment for any of its stock, the power to adopt, amend or repeal bylaws shall be vested in the stockholders entitled to vote; provided, however, that any corporation nay, in its certificate of incorporation, provide that bylaws may be adopted, amended or repealed by the board of directors. The fact that such power has been conferred upon the board of directors shall not divest the stockholders of the power nor limit their power to adopt, amend or repeal the bylaws. |
Amendment of Bylaws. Under the QBCA, the directors may, by resolution, make, amend or repeal any bylaws that regulates the business or affairs of the corporation. Where the directors make, amend or repeal a bylaw, they are required under the QBCA to submit that action to the shareholders at the next meeting of shareholders and the shareholders may confirm, reject or amend that action by simple majority, or ordinary resolution. If the action is rejected by shareholders, or the directors of a corporation do not submit the action to the shareholders at the next meeting of shareholders, the action will cease to be effective, and no subsequent resolution of the directors to make, amend or repeal a bylaw having substantially the same purpose or effect will he effective until it is confirmed. |
|
| Votes on Mergers, Consolidations and Sales of Assets |
||
| Delaware |
Quebec | |
| The DGCL provides that, unless otherwise provided in the certificate of incorporation or bylaws, the adoption of a merger agreement requires the approval of a majority of the outstanding stock of the corporation entitled to vote thereon. |
Under the QBCA, certain extraordinary corporate actions, such as amalgamations (other than with certain affiliated corporations), continuances and sales, leases or exchanges of the property of a corporation if as a result of such alienation the corporation would be unable to retain a significant part of its business activities, and other extraordinary corporate actions such as liquidations, dissolutions and (if ordered by a court) arrangements, are required to be approved by special resolution.
A special resolution is a resolution passed by not less than two-thirds of the votes cast by the shareholders who voted in respect of the resolution or signed by all |
|
- 120 -
Table of Contents
| shareholders entitled to vote on the resolution. In specified cases, a special resolution to approve the extraordinary corporate action is also required to be approved separately by the holders of a class or series of shares, including in certain cases a class or series of shares not otherwise carrying voting rights. |
||
| Dissenters Rights of Appraisal |
||
| Delaware |
Quebec | |
| Under the DGCL, a stockholder of a Delaware corporation generally has the right to dissent flume, merger or consolidation in which the Delaware corporation is participating, subject to specified procedural requirements, including that such dissenting stockholder does not vote in favor of the merger or consolidation. However, the DGCL does not confer appraisal rights, in certain circumstances, including if the dissenting stockholder owns shares traded on a national securities exchange and will receive publicly traded shares in the merger or consolidation. Under the DGCL, a stockholder asserting appraisal rights does not receive any payment for his or her shares until the court determines the fair value or the parties otherwise agree to a value. The costs of the proceeding may be determined by the court and assessed against the parties as the court deems equitable under the circumstances. |
The QBCA provides that shareholders of a corporation are entitled to exercise dissent rights (called the right to demand the repurchase of shares) and to be paid the fair value of their shares in connection with specified matters, including:
any amalgamation with another corporation (other than with certain affiliated corporations);
an amendment to the corporations articles to add, change or remove any provisions restricting or constraining the transfer of shares;
an amendment to the corporations articles to add. change or remove any restriction upon the businesses or businesses that the corporation may carry on;
a continuance under the laws of another jurisdiction;
a sale, lease or exchange of the property of the corporation or of its subsidiaries if, as a result of such alienation, the corporation is unable to retain a significant part of its business activity;
a court order permitting a shareholder to exercise his right to demand the repurchase of his shares in connection with an application to the court for an order approving an arrangement proposed by the corporation;
the carrying out of a going-private transaction; and certain amendments to the articles of a corporation which require a separate class or series vote by a holder of shares of any class or series.
However, a shareholder is not entitled to dissent if an amendment to the articles is effected by a court order approving reorganization or by a court order made in connection with an action for an oppression remedy. |
|
- 121 -
Table of Contents
| Oppression Remedy |
||
| Delaware |
Quebec | |
| The DGCL does not provide for a similar remedy. |
The QBCA provides an oppression remedy (called rectification of abuse of power or iniquity) that enables a court to make any order, whether interim or final, to rectify matters that are oppressive or unfairly prejudicial to the interests of any securityholder, director or officer of the corporation if an application is made to a court by an applicant. An applicant with respect to a corporation means any of the following:
a present or former registered holder or beneficiary of securities of the corporation or any of its affiliates;
a present or former officer or director of the corporation or any of its affiliates; and
any other person who in the discretion of the court has the interest to make the application.
The oppression remedy provides the court with very broad and flexible powers to intervene in corporate affairs to protect shareholders and other complainants. While conduct that is in breach of fiduciary duties of directors or that is contrary to the legal right of a complainant will normally trigger the courts jurisdiction under the oppression remedy, the exercise of that jurisdiction does not depend on a finding of a breach of those legal and equitable rights. Furthermore, the court may order a corporation to pay the interim expenses of an applicant seeking an oppression remedy, but the applicant may be held accountable for interim costs on final disposition of the complaint (as in the case of a derivative action as described in Shareholder Derivative Actions below). |
|
| Shareholder Derivative Actions |
||
| Delaware |
Quebec | |
| Under the DGCL, stockholders may bring derivative actions on behalf of, and for the benefit of the corporation. The plaintiff in a derivative action on behalf of the corporation either must be or have been a stockholder of the corporation at the time of the transaction or must be a stockholder who became a stockholder by operation of law in the transaction regarding which the stockholder complains. A stockholder may not sue derivatively on behalf of the corporation unless the stockholder first makes demand on the corporation that it bring suit and the demand is refused, unless it is shown that making the demand would have been a futile act. |
Under the QBCA, a shareholder of a corporation may apply to a Quebec court for leave to bring an action in the name of, and on behalf of, the corporation or any subsidiary, or to intervene in an existing action to which the corporation or any of its subsidiaries is a party, for the purpose of prosecuting, defending or discontinuing an action on behalf of the corporation or its subsidiary. Under the QBCA, no action may be brought and no intervention in an action may be made unless a court is satisfied that:
the shareholder has given the required 14-day notice to the directors of the corporation or the |
|
- 122 -
Table of Contents
| subsidiary of the shareholders intention to apply to the court if the directors do not bring, diligently prosecute or defend or discontinue the action;
the shareholder is acting in good faith; and
it appears to be in the interests of the corporation or the relevant subsidiary that the action be brought. prosecuted, defended or discontinued.
Under the QBCA, the court in a derivative action may make any order it thinks fit. In addition, under the QBCA, a court may order the corporation or its relevant subsidiary to pay the shareholders interim costs, including reasonable legal fees and disbursements. Although the shareholder may he held accountable for the interim costs on final disposition of the complaint, the shareholder is not required to give security for costs in a derivative action. |
||
| Anti-Takeover and Ownership Provisions |
||
| Delaware |
Quebec | |
| Unless an issuer opts out of the provisions of Section 203 of the DGCL, Section 203 generally prohibits a public Delaware corporation from engaging in a business combination with a holder of 15% or more of the corporations voting stock (as defined in Section 203), referred to as an interested stockholder, for a period of three years after the date of the transaction in which the interested stockholder became an interested stockholder, except as otherwise provided in Section 203. For these purposes, the term business combination includes mergers, assets sales and other similar transactions with an interested stockholder. |
While the QBCA does not contain specific anti- takeover provisions with respect to business combinations, rules and policies of certain Canadian securities regulatory authorities, including Multilateral Instrument 61-101Protection of Minority Security Holders in Special Transactions, or Multilateral Instrument 61-101, contain requirements in connection with, among other things, related party transactions and business combinations, including, among other things, any transaction by which an issuer directly or indirectly engages in the following with a related party: acquires, sells, leases or transfers an asset, acquires the related party, acquires or issues treasury securities, amends the terms of a security if the security is owned by the related party or assumes or becomes subject to a liability or takes certain other actions with respect to debt.
The term related party includes directors, senior officers and holders of more than 10% of the voting rights attached to all outstanding voting securities of the issuer or holders of a sufficient number of any securities of the issuer to materially affect control of the issuer.
Multilateral Instrument 61-101 requires, subject to certain exceptions, the preparation of a formal valuation relating to certain aspects of the transaction and more detailed disclosure in the proxy material sent to security holders in connection with a related |
|
- 123 -
Table of Contents
| party transaction including related to the valuation. Multilateral Instrument 61-101 also requires, subject to certain exceptions, that an issuer not engage in a related party transaction unless the shareholders of the issuer, other than the related parties, approve the transaction by a simple majority of the votes cast. |
- 124 -
Table of Contents
CERTAIN U.S. FEDERAL INCOME TAX CONSIDERATIONS
The following is a summary of certain U.S. federal income tax considerations arising from and relating to the acquisition, ownership, and disposition of our common shares and warrants to a U.S. Holder (as defined below) who acquires such common shares and warrants pursuant to this prospectus. This discussion does not address the tax consequences to a subsequent purchaser of our common shares or warrants. This summary provides only general information and does not purport to be a complete analysis or listing of all potential U.S. federal income tax consequences that may apply to a U.S. Holder as a result of the acquisition, ownership, and disposition of our common shares or warrants. In addition, this summary does not take into account the individual facts and circumstances of any particular U.S. Holder that may affect the U.S. federal income tax consequences applicable to that U.S. Holder. Accordingly, this summary is not intended to be, and should not be construed as, legal or U.S. federal income tax advice with respect to any U.S. Holder. Each U.S. Holder should consult its own tax advisor regarding the U.S. federal, U.S. state and local, and non-U.S. tax consequences arising from or relating to the acquisition, ownership, and disposition of our common shares or warrants.
No legal opinion from U.S. legal counsel or ruling from the Internal Revenue Service, or IRS, has been requested, or will be obtained, regarding the U.S. federal income tax consequences to U.S. Holders of the acquisition, ownership, and disposition of our common shares or warrants. This summary is not binding on the IRS, and the IRS is not precluded from taking a position that is different from, and contrary to, the positions taken in this summary. In addition, because the authorities on which this summary is based are subject to various interpretations, the IRS and the U.S. courts could disagree with one or more of the positions taken in this summary.
Scope of this Disclosure
Authorities
This summary is based on the U.S. Internal Revenue Code of 1986, as amended, or the Code, U.S. Treasury Regulations promulgated thereunder (whether final, temporary or proposed), published IRS rulings, judicial decisions, published administrative positions of the IRS, and the Convention between Canada and the United States of America with Respect to Taxes on Income and on Capital, signed September 26, 1980, as amended (the Canada-U.S. Tax Treaty), in each case, as in effect as of the date of this prospectus. Any of the authorities on which this summary is based could be changed in a material and adverse manner at any time, and any such change could be applied on a retroactive basis. Unless otherwise discussed, this summary does not discuss the potential effects, whether adverse or beneficial, of any proposed legislation.
U.S. Holders
For purposes of this summary, a U.S. Holder is a beneficial owner of common shares or warrants that, for U.S. federal income tax purposes, is (a) an individual who is a citizen or resident of the United States, (b) a corporation, or other entity classified as a corporation for U.S. federal income tax purposes, that is created or organized in or under the laws of the U.S., any state in the United States or the District of Columbia, (c) an estate if the income of such estate is subject to U.S. federal income tax regardless of the source of such income, or (d) a trust if (i) such trust has validly elected to be treated as a U.S. person for U.S. federal income tax purposes or (ii) a U.S. court is able to exercise primary supervision over the administration of such trust and one or more U.S. persons have the authority to control all substantial decisions of such trust.
U.S. Holders Subject to Special U.S. Federal Income Tax Rules Not Addressed
This summary does not address the U.S. federal income tax consequences applicable to U.S. Holders that are subject to special provisions under the Code, including, but not limited to, the following U.S. Holders: (a) U.S. Holders that are tax-exempt organizations, qualified retirement plans, individual retirement accounts, or other tax
- 125 -
Table of Contents
deferred accounts; (b) U.S. Holders that are financial institutions, insurance companies, real estate investment trusts, or regulated investment companies; (c) U.S. Holders that are dealers in securities or currencies or U.S. Holders that are traders in securities that elect to apply a mark-to-market accounting method; (d) U.S. Holders that have a functional currency other than the U.S. dollar; (e) U.S. Holders subject to the alternative minimum tax provisions of the Code; (f) U.S. Holders that own common shares or warrants as part of a straddle, hedging transaction, conversion transaction, integrated transaction, constructive sale, or other arrangement involving more than one position; (g) U.S. Holders that acquired common shares or warrants through the exercise of employee stock options or otherwise as compensation for services; (h) U.S. Holders that hold common shares or warrants other than as a capital asset within the meaning of Section 1221 of the Code; (i) U.S. Holders that beneficially own (directly, indirectly or by attribution) 10% or more of our voting securities or otherwise held 10% or more of our total combined voting power; and (j) U.S. expatriates. U.S. Holders that are subject to special provisions under the Code, including U.S. Holders described above, should consult their own tax advisor regarding the U.S. federal, U.S. federal alternative minimum, U.S. federal estate and gift, U.S. state and local, and non-U.S. tax consequences arising from and relating to the acquisition, ownership, and disposition of the common shares or warrants.
If an entity or arrangement that is classified as a partnership for U.S. federal income tax purposes holds common shares or warrants, the U.S. federal income tax consequences to that partnership and the partners of that partnership generally will depend on the activities of the partnership and the status of the partners. Partners of entities that are classified as partnerships for U.S. federal income tax purposes should consult their own tax advisors regarding the U.S. federal income tax consequences arising from and relating to the acquisition, ownership and disposition of the common shares or warrants.
Tax Consequences Other than U.S. Federal Income Tax Consequences Not Addressed
This summary does not address the U.S. estate and gift, alternative minimum, state, local or non-U.S. tax consequences to U.S. Holders of the acquisition, ownership, and disposition of our common shares or warrants. Each U.S. Holder should consult its own tax advisor regarding the U.S. estate and gift, alternative minimum, state, local and non-U.S. tax consequences arising from and relating to the acquisition, ownership, and disposition of our common shares or warrants.
Apportionment of Purchase Price
For U.S. federal income tax purposes, each U.S. Holder who acquires common shares and warrants pursuant to this prospectus generally must allocate the amount paid between the common shares and the warrants based on the relative fair market value of each on the date of acquisition. The price allocated to each common share and warrant generally will be such holders tax basis in such common share or warrant, as the case may be. We intend to apportion $0.53 to each common share and $0.48 to each warrant. However, the IRS is not bound by this allocation of the purchase price for the common shares and warrants and therefore the IRS or a U.S. court may not respect such allocation.
Each U.S. Holder is advised to consult its own tax advisor regarding the risks associated with an investment in the common shares and warrants and regarding an allocation of the purchase price between the common shares and the warrants.
U.S. Federal Income Tax Considerations of the Acquisition, Ownership, and Disposition of Common Shares
Distributions on Common Shares
Subject to the discussion under Passive Foreign Investment Company Rules below, a U.S. Holder that receives a distribution, including a constructive distribution or a taxable stock distribution, with respect to the
- 126 -
Table of Contents
common shares generally will be required to include the amount of that distribution in gross income as a dividend (without reduction for any Canadian income tax withheld from such distribution) to the extent of our current or accumulated earnings and profits (as computed for U.S. federal income tax purposes). To the extent that a distribution exceeds our current and accumulated earnings and profits, the excess amount will be treated (a) first, as a tax-free return of capital to the extent of a U.S. Holders adjusted tax basis in the common shares with respect to which the distribution is made (resulting in a corresponding reduction in the tax basis of those common shares) and, (b) thereafter, as gain from the sale or exchange of those common shares (see the more detailed discussion at Disposition of Common Shares below). We do not intend to calculate our current or accumulated earnings and profits for U.S. federal income tax purposes and, therefore, will not be able to provide U.S. Holders with that information. U.S. Holders should therefore assume that any distribution by us with respect to our common shares will constitute a dividend. However, U.S. Holders should consult their own tax advisors regarding whether distributions from us should be treated as dividends for U.S. federal income tax purposes. Dividends paid on our common shares generally will not be eligible for the dividends received deduction allowed to corporations under the Code with respect to dividends received from U.S. corporations.
A dividend paid by us generally will be taxed at the preferential tax rates applicable to long-term capital gains if, among other requirements, (a) we are a qualified foreign corporation (as defined below), (b) the U.S. Holder receiving the dividend is an individual, estate, or trust, and (c) the dividend is paid on common shares that have been held by the U.S. Holder for at least 61 days during the 121-day period beginning 60 days before the ex-dividend date (i.e., the first date that a purchaser of the common shares will not be entitled to receive the dividend).
For purposes of the rules described in the preceding paragraph, we generally will be a qualified foreign corporation, or a QFC, if (a) we are eligible for the benefits of the Canada-U.S. Tax Treaty, or (b) our common shares are readily tradable on an established securities market in the United States, within the meaning provided in the Code. However, even if we satisfy one or more of the requirements, we will not be treated as a QFC if we are classified as a PFIC (as discussed below) for the taxable year during which we pay the applicable dividend or for the preceding taxable year. The dividend rules are complex, and each U.S. Holder should consult its own tax advisor regarding the application of those rules to them in their particular circumstances. Even if we satisfy one or more of the requirements, as noted below, there can be no assurance that we will not be a PFIC in the current taxable year, or become a PFIC in the future. Thus, there can be no assurance that we will qualify as a QFC.
Disposition of Common Shares
Subject to the discussion under Passive Foreign Investment Company Rules below, a U.S. Holder will recognize gain or loss on the sale or other taxable disposition of common shares (that is treated as a sale or exchange for U.S. federal income tax purposes) equal to the difference, if any, between (a) the U.S. dollar value of the amount realized on the date of the sale or disposition and (b) the U.S. Holders adjusted tax basis (determined in U.S. dollars) in the common shares sold or otherwise disposed of. Any such gain or loss generally will be capital gain or loss, which will be long-term capital gain or loss if the common shares are held for more than one year. Each U.S. Holder should consult its own tax advisor as to the tax treatment of dispositions of common shares in exchange for Canadian dollars.
Preferential tax rates apply to long-term capital gains of a U.S. Holder that is an individual, estate, or trust. There are currently no preferential tax rates for long-term capital gains of a U.S. Holder that is a corporation. Deductions for capital losses are subject to complex limitations.
Passive Foreign Investment Company Rules
If we are or become a PFIC, the preceding sections of this summary may not describe the U.S. federal income tax consequences to U.S. Holders of the acquisition, ownership, and disposition of our common shares.
- 127 -
Table of Contents
Passive Foreign Investment Company Status.
Special, generally unfavorable, rules apply to the ownership and disposition of the stock of a PFIC. For U.S. federal income tax purposes, a non-U.S. corporation is classified as a PFIC for each taxable year in which either:
| | at least 75% of its gross income is passive income (referred to as the income test); or |
| | at least 50% of the average value of its assets is attributable to assets that produce passive income or are held for the production of passive income (referred to as the asset test). |
Passive income includes the following types of income:
| | dividends, royalties, rents, annuities, interest, and income equivalent to interest; and |
| | net gains from the sale or exchange of property that gives rise to dividends, interest, royalties, rents, or annuities and certain gains from the commodities transactions. |
In determining whether we are a PFIC, we will be required to take into account a pro rata portion of the income and assets of each corporation in which we own, directly or indirectly, at least 25% by value.
As described above, PFIC status of a non-U.S. corporation for a taxable year depends on the relative values of certain categories of assets and the relative amount of certain kinds of income. Therefore, our status as a PFIC for any given taxable year depends upon the financial results for such year and upon relative valuations, which are subject to change and beyond our ability to predict or control. Based on our most recent financial statements and projections and given uncertainty regarding the composition of our future income and assets, there is a significant risk that we may be classified as a PFIC for the fiscal year ending March 31, 2018 and possibly in subsequent years. However, PFIC status is fundamentally factual in nature, depends on the application of complex U.S. federal income tax rules (which are subject to differing interpretations), generally cannot be determined until the close of the taxable year in question and is determined annually. Accordingly, there can be no assurance that we will not be a PFIC in our 2018 taxable year or subsequent years. The PFIC rules are complex, and each U.S. Holder should consult its tax advisor regarding the application of the PFIC rules to us.
Default PFIC Rules Under Section 1291 of the Code.
Generally, if we are or have been treated as a PFIC for any taxable year during a U.S. Holders holding period of common shares, subject to the special rules described below applicable to a U.S. Holder who makes a Mark-to-Market Election or a QEF Election (each as defined below), any excess distribution with respect to the common shares would be allocated ratably over the U.S. Holders holding period. The amounts allocated to the taxable year of the excess distribution and to any year before we became a PFIC would be taxed as ordinary income. The amount allocated to each other taxable year would be subject to tax at the highest rate in effect for individuals or corporations in that taxable year, as appropriate, and an interest charge would be imposed on the amount allocated to that taxable year. Distributions made in respect of common shares during a taxable year will be excess distributions to the extent they exceed 125% of the average of the annual distributions on common shares received by the U.S. Holder during the preceding three taxable years or the U.S. Holders holding period, whichever is shorter. In addition, dividends generally will not be qualified dividend income if we are a PFIC in the taxable year of payment or the preceding year.
Generally, if we are treated as a PFIC for any taxable year during which a U.S. Holder owns common shares, any gain on the disposition of the common shares would be treated as an excess distribution and would be allocated ratably over the U.S. Holders holding period and subject to taxation in the same manner as described in the preceding paragraph, and would not be eligible for the preferential long-term capital gains rate.
- 128 -
Table of Contents
Certain elections (including the Mark-to-Market Election and the QEF Election, as defined and discussed below) may sometimes be used to mitigate the adverse impact of the PFIC rules on U.S. Holders, but these elections may accelerate the recognition of taxable income and have other adverse results.
U.S. HOLDERS ARE URGED TO CONSULT THEIR TAX ADVISORS REGARDING THE POSSIBLE APPLICABILITY OF THE PFIC RULES TO SHARES AND WARRANTS ACQUIRED PURSUANT TO THIS PROSPECTUS, AND THE AVAILABILITY OF MAKING A QEF OR MARK-TO-MARKET ELECTION TO MITIGATE ADVERSE U.S. TAX CONSEQUENCES OF HOLDING SHARES OF A PFIC.
QEF Election.
A U.S. Holder of common shares in a PFIC generally would not be subject to the PFIC rules discussed above if the U.S. Holder had made a timely and effective election (a QEF Election) to treat us as a qualified electing fund (a QEF). Instead, such U.S. Holder would be subject to U.S. federal income tax on its pro rata share of our (i) net capital gain, which would be taxed as long-term capital gain to such U.S. Holder, and (ii) ordinary earnings, which would be taxed as ordinary income to such U.S. Holder, in each case regardless of whether such amounts are actually distributed to such U.S. Holder. However, a U.S. Holder that makes a QEF Election may, subject to certain limitations, elect to defer payment of current U.S. federal income tax on such amounts, subject to an interest charge. If such U.S. Holder is not a corporation, any such interest paid will be treated as personal interest, which is not deductible.
A U.S. Holder that makes a timely and effective QEF Election generally (a) may receive a tax-free distribution from us to the extent that such distribution represents our earnings and profits that were previously included in income by such U.S. Holder because of such QEF Election and (b) will adjust such U.S. Holders tax basis in the common shares to reflect the amount included in income or allowed as a tax-free distribution because of such QEF Election. In addition, for U.S. federal income tax purposes, a U.S. Holder that makes a timely QEF Election generally will recognize capital gain or loss on the sale or other taxable disposition of the common shares.
A QEF Election will be treated as timely if such QEF Election is made for the first taxable year in the U.S. Holders holding period for the common shares in which we are a PFIC. A U.S. Holder may make a timely QEF election by filing the appropriate QEF Election documents at the time such U.S. Holder files a U.S. federal income tax return for such first year. If a U.S. Holder makes a QEF Election after the first taxable year in the U.S. Holders holding period for the common shares in which we are a PFIC, then, in addition to filing the QEF Election documents, a U.S. Holder may elect to recognize gain (which will be taxed under the rules discussed under Default PFIC Rules Under Section 1291 of the Code) as if the common shares were sold on the qualification date. The qualification date is the first day of the first taxable year in which we are a QEF with respect to such U.S. Holder. The election to recognize such gain can only be made if such U.S. Holders holding period for the common shares includes the qualification date. By electing to recognize such gain, such U.S. Holder will be deemed to have made a timely QEF Election. In addition, under very limited circumstances, it is possible that a U.S. Holder might make a retroactive QEF Election if such U.S. Holder failed to file the QEF Election documents in a timely manner. If a U.S. Holder fails to make a QEF Election for the first taxable year in the U.S. Holders holding period for the common shares in which we are a PFIC and does not elect to recognize gain as if the common shares were sold on the qualification date, such holder will not be treated as having made a timely QEF election and will continue to be subject to the special adverse taxation rules discussed above under Default PFIC Rules Under Section 1291 of the Code.
A QEF Election will apply to the taxable year for which such QEF election is made and to all subsequent taxable years, unless such QEF Election is invalidated or terminated or the IRS consents to revocation of such QEF Election. If a U.S. Holder makes a QEF Election and, in a subsequent taxable year, we cease to be a PFIC, the QEF Election will remain in effect (although it will not be applicable) during those taxable years in which we are
- 129 -
Table of Contents
not a PFIC. Accordingly, if we become a PFIC in another subsequent taxable year, the QEF Election will be effective and the U.S. Holder will be subject to the rules described above during any such subsequent taxable year in which we qualify as a PFIC.
A U.S. Holder cannot make and maintain a valid QEF Election unless we provide certain U.S. tax information necessary to make such an election. On an annual basis, we intend to use commercially reasonable efforts to make available to U.S. Holders that acquire common shares pursuant to this prospectus, upon their written request (a) timely information as to our status as a PFIC, and (b) for each year in which we are a PFIC, information and documentation that a U.S. Holder making a QEF Election with respect to us is required to obtain for U.S. federal income tax purposes. Each U.S. Holder should consult its own tax advisor regarding the availability of, and procedure for making, a QEF Election with respect to us.
Mark-to-Market Election.
A U.S. Holder of common shares in a PFIC would not be subject to the PFIC rules discussed above under Default PFIC Rules Under Section 1291 of the Code if the U.S. Holder had made a timely and effective election to mark the PFIC common shares to market (a Mark-to-Market Election).
A U.S. Holder may make a Mark-to-Market Election with respect to the common shares only if such shares are marketable stock. Such shares generally will be marketable stock if they are regularly traded on a qualified exchange, which is defined as (a) a national securities exchange that is registered with the Securities and Exchange Commission, (b) the national market system established pursuant to section 11A of the Exchange Act of 1934, or (c) a non-U.S. securities exchange that is regulated or supervised by a governmental authority of the country in which the market is located, provided that (i) such non-U.S. exchange has trading volume, listing, financial disclosure, surveillance, and other requirements, and the laws of the country in which such non-U.S. exchange is located, together with the rules of such non-U.S. exchange, ensure that such requirements are actually enforced and (ii) the rules of such non-U.S. exchange ensure active trading of listed stocks. Our common shares will generally be treated as regularly traded in any calendar year in which more than a de minimis quantity of common shares is traded on a qualified exchange for at least 15 days during each calendar quarter. Each U.S. Holder should consult its own tax advisor with respect to the availability of a Mark-to-Market Election with respect to the common shares.
In general, a U.S. Holder that makes a timely Mark-to-Market Election with respect to the common shares will include in ordinary income, for each taxable year in which we are a PFIC, an amount equal to the excess, if any, of (a) the fair market value of the common shares as of the close of such taxable year over (b) such U.S. Holders tax basis in such shares. A U.S. Holder that makes a Mark-to-Market Election will be allowed a deduction in an amount equal to the lesser of (a) the excess, if any, of (i) such U.S. Holders adjusted tax basis in the common shares over (ii) the fair market value of such shares as of the close of such taxable year or (b) the excess, if any, of (i) the amount included in ordinary income because of such Mark-to-Market Election for prior taxable years over (ii) the amount allowed as a deduction because of such Mark-to-Market Election for prior taxable years. If a U.S. Holder makes a Mark-to-Market Election after the first taxable year in which we are a PFIC and such U.S. Holder has not made a timely QEF Election with respect to us, the PFIC rules described above under Default PFIC Rules Under Section 1291 of the Code will apply to certain dispositions of, and distributions on, the common shares, and the U.S. Holders mark-to-market income for the year of the election. If we were to cease being a PFIC, a U.S. Holder that marked its common shares to market would not include mark-to-market gain or loss with respect to its common shares for any taxable year that we were not a PFIC.
A U.S. Holder that makes a Mark-to-Market Election generally will also adjust such U.S. Holders tax basis in his common shares to reflect the amount included in gross income or allowed as a deduction because of such Mark-to-Market Election. In addition, upon a sale or other taxable disposition of the common shares subject to a Mark-to-Market Election, any gain or loss on such disposition will be ordinary income or loss (to the extent that such loss does not to exceed the excess, if any, of (a) the amount included in ordinary income because of such
- 130 -
Table of Contents
Mark-to-Market Election for prior taxable years over (b) the amount allowed as a deduction because of such Mark-to-Market Election for prior taxable years). A Mark-to-Market Election applies to the taxable year in which such Mark-to-Market Election is made and to each subsequent taxable year, unless the common shares cease to be marketable stock or the IRS consents to revocation of such election. Each U.S. Holder should consult its own tax advisor regarding the availability of, and procedure for making, a Mark-to-Market Election with respect to the common shares.
Reporting. If we were to be treated as a PFIC in any taxable year, a U.S. Holder will generally be required to file an annual report with the IRS containing such information as the U.S. Treasury Department may require.
Each U.S. Holder should consult its own tax advisor regarding our potential status as a PFIC, the possible effect of the PFIC rules to such holder and information reporting required if we were a PFIC, as well as the availability of any election that may be available to the holder to mitigate adverse U.S. federal income tax consequences of holding shares in a PFIC.
U.S. Federal Income Tax Consequences of the Acquisition, Ownership, Disposition, and Exercise of Warrants
Sale or Other Disposition of Warrants
If we are not treated as a PFIC at any time during which a U.S. Holder owns warrants, such U.S. Holder will recognize gain or loss on a sale or other taxable disposition of the warrants (other than by exercise) equal to the difference, if any, between (a) the U.S. dollar value of the amount realized on the date of such sale or disposition and (b) such U.S. Holders adjusted tax basis (determined in U.S. dollars) in the warrants sold or otherwise disposed of. Any such gain or loss generally will be capital gain or loss, which will be long-term capital gain or loss if such warrants are held for more than one year.
If a U.S. Holders holding period for its warrants includes any portion of a taxable year for which we are a PFIC, then any gain on a sale or other taxable disposition of the warrants (other than by exercise) generally will be taxed as an excess distribution under the PFIC rules discussed above under U.S. Federal Income Tax Considerations of the Acquisition, Ownership, and Disposition of Common SharesPassive Foreign Investment Company Rules. Any loss from such sale or disposition will generally be treated as a capital loss, which will be long-term capital loss if such warrants are held for more than one year. Neither the QEF Election nor the Mark-to-Market Election is available with respect to the Warrants.
Exercise of Warrants
Except with respect to a cashless exercise of warrants, upon the exercise of warrants and related receipt of common shares, a U.S. Holder will generally not recognize gain or loss unless cash is received in lieu of the issuance of a fractional common share, and will have a tax basis for the common shares so acquired equal to the sum of the exercise price paid by such holder and such holders adjusted tax basis for the warrants, less any amount attributable to any fractional share with respect to which such holder received cash. The receipt of cash in lieu of a fractional common share should generally be treated as if the U.S. holder received the fractional share and then received such cash in redemption of such fractional share. Such redemption should generally result in capital gain or loss equal to the difference between the amount of cash received and such holders adjusted tax basis in the common share that is allocable to the fractional share. Any such gain will generally be treated as an excess distribution under the PFIC rules discussed above.
In general, a U.S. Holders holding period for common shares acquired upon exercise of warrants will not include the period during which such holder owned the warrants. However, if we have been a PFIC for any taxable year all or a portion of which includes the period the U.S. Holder owned the warrants, then solely for purposes of applying the PFIC rules to the common shares acquired upon exercise of such warrants, the holding period will include the period during which the U.S. Holder owned the warrants.
- 131 -
Table of Contents
The tax treatment of a cashless exercise is uncertain and could materially differ from the consequences upon the exercise of a warrant described above. U.S. Holders are urged to consult their tax advisors with respect to the tax consequences of a cashless exercise.
Expiration of Warrants Without Exercise
Upon the lapse or expiration of a warrant, a U.S. Holder will generally recognize a loss in an amount equal to such U.S. Holders tax basis in the warrant. Any such loss generally will be a capital loss and will be long-term capital loss if the warrant was held for more than one year. The deductibility of a capital loss may be subject to limitations.
Constructive Dividends
Under Section 305 of the Code, an adjustment to the number of shares of our common shares that will be issued on the exercise of the warrants, an adjustment to the exercise price of our warrants, or the failure to make adjustments to the exercise price upon the occurrence of certain events, may be treated as a constructive distribution to a U.S. Holder of the warrants if, and to the extent that, such adjustment (or failure to adjust) has the effect of increasing such U.S. Holders proportionate interest in our earnings and profits or assets, depending on the circumstances of such adjustment (for example, if such adjustment is to compensate for a distribution of cash or other property to our shareholders). Adjustments to the exercise price of a warrant made pursuant to a bona fide reasonable adjustment formula that has the effect of preventing dilution of the interest of the holders of our warrants should generally not result in a constructive distribution. U.S. Holders should consult their own tax advisers with respect to the tax consequences of any exercise price adjustment.
Receipt of Foreign Currency
The amount of a distribution paid in Canadian dollars or Canadian dollar proceeds received on the sale or other taxable disposition of common shares or warrants will generally be equal to the U.S. dollar value of the currency on the date of receipt. If any Canadian dollars received with respect to the common shares or warrants are later converted into U.S. dollars, U.S. Holders may realize gain or loss on the conversion. Any gain or loss generally will be treated as ordinary income or loss and generally will be from sources within the United States for U.S. foreign tax credit purposes. Each U.S. Holder should consult its own tax advisor concerning the possibility of foreign currency gain or loss if any such currency is not converted into U.S. dollars on the date of receipt.
Foreign Tax Credit
Subject to certain limitations, a U.S. Holder who pays (whether directly or through withholding) Canadian or other non-U.S. income tax with respect to the common shares or warrants may be entitled, at the election of the U.S. Holder, to receive either a deduction or a credit for Canadian or other non-U.S. income tax paid. Dividends paid on common shares generally will constitute income from sources outside the United States. The foreign tax credit rules (including the limitations with respect thereto) are complex, and each U.S. Holder should consult its own tax advisor regarding the foreign tax credit rules, having regard to such holders particular circumstances.
Information Reporting; Backup Withholding
Generally, information reporting and backup withholding will apply to distributions on, and the payment of proceeds from the sale or other taxable disposition of, the common shares or warrants unless (i) the U.S. Holder is a corporation or other exempt entity, or (ii) in the case of backup withholding, the U.S. Holder provides a correct taxpayer identification number and certifies that the U.S. Holder is not subject to backup withholding.
Backup withholding is not an additional tax. Any amount withheld generally will be creditable against a U.S. Holders U.S. federal income tax liability or refundable to the extent that it exceeds such liability provided the required information is provided to the IRS in a timely manner.
- 132 -
Table of Contents
In addition, certain categories of U.S. Holders must file information returns with respect to their investment in a non-U.S. corporation. For example, certain U.S. Holders must file IRS Form 8938 with respect to certain specified foreign financial assets (such as the common shares and warrants) with an aggregate value in excess of US$50,000 (and, in some circumstances, a higher threshold). Failure to do so could result in substantial penalties and in the extension of the statute of limitations with respect to such holders U.S. federal income tax returns. Each U.S. Holder should consult its own tax advisor regarding application of the information reporting and backup withholding rules to it in connection with an investment in our common shares or warrants.
Medicare Contribution Tax
U.S. Holders that are individuals, estates or certain trusts generally will be subject to a 3.8% Medicare contribution tax on, among other things, dividends on, and capital gains from the sale or other taxable disposition of, common shares or warrants, subject to certain limitations and exceptions. Each U.S. Holder should consult its own tax advisor regarding possible application of this additional tax to income earned in connection with an investment in our common shares or warrants.
- 133 -
Table of Contents
CERTAIN CANADIAN FEDERAL INCOME TAX CONSIDERATIONS
The following is, as of the date hereof, a summary of the principal Canadian federal income tax considerations generally applicable under the Income Tax Act (Canada) and the regulations promulgated thereunder (collectively the Tax Act), to a purchaser who acquires, as beneficial owner, the offered shares and warrants under this offering, and who, for purposes of the Tax Act and at all relevant times, (i) is not, and is not deemed to be, resident in Canada, (ii) holds the offered shares and warrants as capital property, (iii) deals at arms length with, and is not affiliated with, Acasti or the Underwriters, and (iv) does not use or hold and will not be deemed to use or hold, the offered shares and warrants in a business carried on in Canada (a Non-Resident Holder). Special rules, which are not discussed in this summary, may apply to an insurer that carries on an insurance business in Canada and elsewhere.
This summary is based upon the provisions of the Tax Act in force as of the date hereof, all specific proposals to amend the Tax Act that have been publicly announced by or on behalf of the Minister of Finance (Canada) prior to the date hereof (the Proposed Amendments), the Canada-United States Tax Convention (1980), as amended (the Canada-U.S. Tax Treaty), and an understanding of the current administrative policies and assessing practices of the Canada Revenue Agency (the CRA), published in writing by it prior to the date hereof. This summary assumes the Proposed Amendments will be enacted in the form proposed. However, no assurance can be given that the Proposed Amendments will be enacted in their current form, or at all. This summary is not exhaustive of all possible Canadian federal income tax considerations and, except for the Proposed Amendments, does not take into account or anticipate any changes in the law or any changes in the CRAs administrative policies or assessing practices, whether by legislative, governmental or judicial action or decision, nor does it take into account or anticipate any other federal or any provincial, territorial or foreign tax considerations, which may differ significantly from those discussed herein.
This summary is not applicable to a Non-Resident Holder who makes or has made a functional currency reporting election; or that has entered or enters into a derivative forward agreement with respect to the offered shares (each as defined in the Tax Act), including shares acquired by the exercise of the warrants, or the warrants. Any such Non-Resident Holder should consult its own tax advisor with respect to an investment in the offered shares and warrants. This summary is of a general nature only and is not intended to be, nor should it be construed to be, legal or tax advice to any prospective purchaser or holder of the offered shares and warrants, and no representations with respect to the income tax consequences to any prospective purchaser or holder are made. Consequently, prospective purchasers or holders of the offered shares and warrants should consult their own tax advisors with respect to their particular circumstances.
A Non-Resident Holder who acquires the offered shares and warrants will be required to allocate the purchase price between the offered shares and the warrants on a reasonable basis in order to determine their respective costs for purposes of the Tax Act. Non-Resident Holders should consult their own tax advisers in this regard.
Currency Conversion
Generally, for purposes of the Tax Act, all amounts relating to the acquisition, holding or disposition of the offered shares and warrants must be converted into Canadian dollars based on the exchange rates as determined in accordance with the Tax Act. The amounts subject to withholding tax and any capital gains or capital losses realized by a Non-Resident Holder may be affected by fluctuations in the Canadian-U.S. dollar exchange rate.
Dispositions
A Non-Resident Holder will not be subject to tax under the Tax Act on the exercise of a warrant or on a disposition of an offered share or warrant, unless the offered share constitutes taxable Canadian property (as defined in the Tax Act) of the Non-Resident Holder at the time of disposition and the Non-Resident Holder is not entitled to relief under an applicable income tax treaty or convention.
- 134 -
Table of Contents
Provided the offered shares are listed on a designated stock exchange, as defined in the Tax Act (which currently includes the TSX and NASDAQ) at the time of disposition, the offered shares will generally not constitute taxable Canadian property of a Non-Resident Holder at that time, unless at any time during the 60-month period immediately preceding the disposition the following two conditions are satisfied concurrently: (i) (a) the Non-Resident Holder; (b) persons with whom the Non-Resident Holder did not deal at arms length; (c) partnerships in which the Non-Resident Holder or a person described in (b) holds a membership interest directly or indirectly through one or more partnerships; or (d) any combination of the persons and partnerships described in (a) through (c), owned 25% or more of the issued shares of any class or series of the shares of Acasti; and (ii) more than 50% of the fair market value of the shares of Acasti was derived directly or indirectly from one or any combination of: real or immovable property situated in Canada, Canadian resource properties, timber resource properties (each as defined in the Tax Act), and options in respect of, or interests in or for civil law rights in, such properties. Notwithstanding the foregoing, in certain circumstances set out in the Tax Act, the offered shares could be deemed to be taxable Canadian property. Even if the offered shares are taxable Canadian property to a Non-Resident Holder, such Non-Resident Holder may be exempt from tax under the Tax Act on the disposition of such common shares by virtue of an applicable income tax treaty or convention. A Non Resident Holder contemplating a disposition of offered shares that may constitute taxable Canadian property should consult a tax advisor prior to such disposition.
Dividends
Dividends paid or credited on the offered shares (including shares acquired by the exercise of a warrant) or deemed to be paid or credited on the offered shares to a Non-Resident Holder will be subject to Canadian withholding tax under the Tax Act at the rate of 25%, although such rate may be reduced under the provisions of an applicable income tax convention between Canada and the Non-Resident Holders country of residence. For example, under the Canada-U.S. Tax Treaty, the applicable rate of Canadian withholding tax is generally reduced to 15%.
- 135 -
Table of Contents
ENFORCEABILITY OF CIVIL LIABILITIES
We are incorporated under the laws of the Province of Quebec. Substantially all of our assets are located outside the United States. In addition, several of our directors and officers are nationals and/or residents of countries other than the United States, and all or a substantial portion of such persons assets may be located outside the United States. As a result, it may be difficult for investors to effect service of process within the United States upon us or such persons or to enforce against them or against us, judgments obtained in United States courts, including judgments predicated upon the civil liability provisions of the securities laws of the United States or any state thereof. In addition, investors should not assume that the courts of Canada (i) would enforce judgments of U.S. courts obtained in actions against us, our officers or directors, or other said persons, predicated upon the civil liability provisions of the U.S. federal securities laws or other laws of the United States or (ii) would enforce, in original actions, liabilities against us or such directors, officers or experts predicated upon the United States federal securities laws or any securities or other laws of any state or jurisdiction of the United States.
Notwithstanding this, we have also been advised by Osler, Hoskin & Harcourt LLP, that there is doubt whether an action could be brought in Canada in the first instance on the basis of liability predicated solely upon United States federal securities laws.
We have appointed C T Corporation System as our agent to receive service of process with respect to any action brought against us in the United States.
- 136 -
Table of Contents
The financial statements of Acasti as at March 31, 2017 and February 29, 2016, and for the thirteen-month period ended March 31, 2017 and the years ended February 29, 2016 and February 28, 2015, have been included herein and in the registration statement in reliance upon the report of KPMG LLP, independent registered public accounting firm, appearing elsewhere herein and upon the authority of said firm as experts in accounting and auditing.
The audit report covering those financial statements contains an emphasis of matter paragraph that states that Acasti has incurred operating losses and negative cash flows from operations since inception, that its current assets as at March 31, 2017 are projected to be significantly less than needed and that its future operations are dependent on obtaining additional financing, which, along with other matters as set forth in 2(c) in the financial statements, indicate the existence of a material uncertainty that casts substantial doubt about Acastis ability to continue as a going concern. The financial statements do not include any adjustments that may be necessary if the going concern basis was not appropriate. The audit report also contains an other matter paragraph that states that the financial statements of Acasti as at February 28, 2017 and for the twelve-month and one-month periods ended February 28, 2017 and March 31, 2017 respectively are unaudited and we do not express an opinion on them.
- 137 -
Table of Contents
The validity of the common shares being offered by this prospectus and other legal matters concerning this offering relating to Canadian law will be passed upon for us by Osler, Hoskin & Harcourt LLP, Montreal, Quebec, Canada. Certain legal matters in connection with this offering relating to U.S. law will be passed upon for us by Osler, Hoskin & Harcourt LLP, New York, New York. Certain legal matters in connection with this offering will be passed upon for the underwriters by Schiff Hardin LLP, Washington, DC.
- 138 -
Table of Contents
Subject to the following paragraph, there is no law or governmental decree or regulation in Canada that restricts the export or import of capital, or affects the remittance of dividends, interest or other payments to non-resident holders of our subordinate voting shares, other than withholding tax requirements.
There is no limitation imposed by Canadian law or by our Articles or our other charter documents on the right of a non-resident to hold or vote voting shares, other than as provided by the Investment Canada Act (Canada), or Investment Canada Act, the North American Free Trade Agreement Implementation Act (Canada), or North American Free Trade Agreement, and the World Trade Organization Agreement Implementation Act. The Investment Canada Act requires notification and, in certain cases, advance review and approval by the Government of Canada of an investment to establish a new Canadian business by a non-Canadian or of the acquisition by a non-Canadian of control of a Canadian business, all as defined in the Investment Canada Act. Generally, the threshold for review will be higher in monetary terms for a member of the World Trade Organization or North American Free Trade Agreement.
Any remittances of dividends to United States residents and to other non-residents are, however, subject to withholding tax. See the sections of this prospectus entitled Certain U.S. Federal Income Tax Considerations and Certain Canadian Federal Income Tax Considerations.
- 139 -
Table of Contents
We are subject to the informational requirements of the Exchange Act, and we file reports and other information with the SEC. You may read and copy any of our reports and other information at, and obtain copies upon payment of prescribed fees from, the Public Reference Room maintained by the SEC at 100 F Street, N.E., Washington, DC 20549. In addition, the SEC maintains a web site that contains reports and other information regarding registrants that file electronically with the SEC at http://www.sec.gov. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330.
As a foreign private issuer, we are exempt under the Exchange Act from, among other things, certain rules prescribing the furnishing and content of proxy statements, and our executive officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we are not required under the Exchange Act to file periodic reports and financial statements with the SEC as frequently or as promptly as U.S. companies whose securities are registered under the Exchange Act.
We are also subject to the full informational requirements of the securities commissions in all provinces of Canada, and you are also invited to read and copy any reports, statements or other information, other than confidential filings, that we file with the Canadian provincial securities commissions. These filings are also electronically available from the Canadian System for Electronic Document Analysis and Retrieval (SEDAR) at www.sedar.com, the Canadian equivalent of the SECs electronic document gathering and retrieval system.
We maintain a corporate website at http://www.acastipharma.com. Information contained on, or that can be accessed through, our website does not constitute a part of this prospectus.
- 140 -
Table of Contents
INDEX TO THE FINANCIAL STATEMENTS
| Unaudited Interim Condensed Financial Statements |
||||
| Interim Statements of Financial Position as of September 30, 2017 and August 31, 2016 |
F-4 | |||
| F-5 | ||||
| F-6 | ||||
| F-7 | ||||
| F-8 | ||||
| Annual Financial Statements |
||||
| Independent Auditors Report of Registered Public Accounting Firm |
F-20 | |||
| Statements of Financial Position as of March 31, 2017, February 28, 2017 and February 29, 2016 |
F-22 | |||
| F-23 | ||||
| F-24 | ||||
| F-26 | ||||
| F-27 | ||||
F-1
Table of Contents
Interim Financial Statements of
(Unaudited)
ACASTI PHARMA INC.
Three-month and six-month periods ended September 30, 2017 and August 31, 2016
F-2
Table of Contents
ACASTI PHARMA INC.
Interim Financial Statements
(Unaudited)
Three-month and six-month periods ended September 30, 2017 and August 31, 2016
Financial Statements
| F-4 | ||||
| F-5 | ||||
| F-6 | ||||
| F-7 | ||||
| F-8 | ||||
F-3
Table of Contents
Acasti Pharma inc.
Interim Statements of Financial Position
(Unaudited)
As at September 30, 2017 and March 31, 2017
| Notes | September 30, 2017 | March 31, 2017 | ||||||||||
| (thousands of Canadian dollars) | $ | $ | ||||||||||
| Assets |
||||||||||||
| Current assets: |
||||||||||||
| Cash and cash equivalents |
5,329 | 9,772 | ||||||||||
| Receivables |
243 | 206 | ||||||||||
| Prepaid expenses |
280 | 303 | ||||||||||
| 5,852 | 10,281 | |||||||||||
| Equipment |
2,678 | 2,787 | ||||||||||
| Intangible assets |
11,227 | 12,388 | ||||||||||
| Total assets |
19,757 | 25,456 | ||||||||||
| Liabilities and Equity |
||||||||||||
| Current liabilities: |
||||||||||||
| Trade and other payables |
3,323 | 2,126 | ||||||||||
| Payable to parent corporation |
68 | 12 | ||||||||||
| 3,391 | 2,138 | |||||||||||
| Derivative warrant liabilities |
5 | 51 | 209 | |||||||||
| Unsecured convertible debentures |
1,509 | 1,406 | ||||||||||
| Total liabilities |
4,951 | 3,753 | ||||||||||
| Equity: |
||||||||||||
| Share capital |
66,633 | 66,576 | ||||||||||
| Other equity |
309 | 309 | ||||||||||
| Contributed surplus |
6,024 | 5,693 | ||||||||||
| Deficit |
(58,160 | ) | (50,875 | ) | ||||||||
| Total equity |
14,806 | 21,703 | ||||||||||
| Commitments and contingencies |
11 | |||||||||||
| Total liabilities and equity |
19,757 | 25,456 | ||||||||||
See accompanying notes to unaudited interim financial statements.
F-4
Table of Contents
ACASTI PHARMA INC.
Interim Statements of Earnings and Comprehensive Loss
(Unaudited)
Three-month and six-month periods ended September 30, 2017 and August 31, 2016
| Three-month periods ended | Six-month periods ended | |||||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||
| September 30, 2017 | August 31, 2016 | September 30, 2017 | August 31, 2016 | |||||||||||||||||||||
| (thousands of Canadian dollars, except per share data) |
Notes | $ | $ | $ | $ | |||||||||||||||||||
| Research and development expenses, net |
7 | (3,349) | (1,594) | (5,331) | (3,987) | |||||||||||||||||||
| General and administrative expenses |
(1,036) | (856) | (1,853) | (1,422) | ||||||||||||||||||||
| Loss from operating activities |
(4,385) | (2,450) | (7,184) | (5,409) | ||||||||||||||||||||
| Financial (expenses) income |
8 | (146) | 55 | (259) | (173) | |||||||||||||||||||
| Change in fair value of warrant liabilities |
5 | 24 | 66 | 158 | 98 | |||||||||||||||||||
| Net financial income (expenses) |
(122) | 121 | (101) | (75) | ||||||||||||||||||||
| Net loss and total comprehensive loss |
(4,507) | (2,329) | (7,285) | (5,484) | ||||||||||||||||||||
| Basic and diluted loss per share |
(0.31) | (0.22) | (0.49) | (0.51) | ||||||||||||||||||||
| Weighted average number of shares outstanding |
14,723,995 | 10,712,038 | 14,717,693 | 10,712,038 | ||||||||||||||||||||
See accompanying notes to unaudited interim financial statements
F-5
Table of Contents
ACASTI PHARMA INC.
Interim Statements of Changes in Equity
(Unaudited)
Six-month periods ended September 30, 2017 and August 31, 2016
| Share capital | Other | Contributed | ||||||||||||||||||||||||
| Notes | Number | Dollar | equity | surplus | Deficit | Total | ||||||||||||||||||||
| (thousands of Canadian dollars) | $ | $ | $ | $ | $ | |||||||||||||||||||||
| Balance, March 31, 2017 |
14,702,556 | 66,576 | 309 | 5,693 | (50,875) | 21,703 | ||||||||||||||||||||
| Net loss and total comprehensive loss for the period |
| | | | (7,285) | (7,285) | ||||||||||||||||||||
| 14,702,556 | 66,576 | 309 | 5,693 | (58,160) | 14,418 | |||||||||||||||||||||
| Transactions with owners, recorded directly in equity |
||||||||||||||||||||||||||
| Contributions by and distributions to equity holders |
||||||||||||||||||||||||||
| Share-based payment transactions |
9 | | | | 331 | | 331 | |||||||||||||||||||
| Issuance of shares for payment of interest on convertible debentures |
6(a) | 33,381 | 57 | | | | 57 | |||||||||||||||||||
| Total contributions by and distributions to equity holders |
33,381 | 57 | | 331 | | 388 | ||||||||||||||||||||
| Balance at September 30, 2017 |
14,735,937 | 66,633 | 309 | 6,024 | (58,160) | 14,806 | ||||||||||||||||||||
| Share capital | Other | Contributed | ||||||||||||||||||||||||
| Notes | Number | Dollar | equity | surplus | Deficit | Total | ||||||||||||||||||||
| (thousands of Canadian dollars) | $ | $ | $ | $ | $ | |||||||||||||||||||||
| Balance, February 29, 2016 |
10,712,038 | 61,973 | | 4,875 | (39,628) | 27,220 | ||||||||||||||||||||
| Net loss and total comprehensive loss for the period |
| | | | (5,484) | (5,484) | ||||||||||||||||||||
| 10,712,038 | 61,973 | | 4,875 | (45,112) | 21,736 | |||||||||||||||||||||
| Transactions with owners, recorded directly in equity |
||||||||||||||||||||||||||
| Contributions by and distributions to equity holders |
||||||||||||||||||||||||||
| Share-based payment transactions |
9 | | | | 275 | | 275 | |||||||||||||||||||
| Total contributions by and distributions to equity holders |
| | | 275 | | 275 | ||||||||||||||||||||
| Balance at August 31, 2016 |
10,712,038 | 61,973 | | 5,150 | (45,112) | 22,011 | ||||||||||||||||||||
See accompanying notes to unaudited interim financial statements.
F-6
Table of Contents
ACASTI PHARMA INC.
Interim Statements of Cash Flows
(Unaudited)
Three-month and six-month periods ended September 30, 2017 and August 31, 2016
| Three-month periods ended | Six-month periods ended | |||||||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| Notes | September 30, 2017 | August 31, 2016 | September 30, 2017 | August 31, 2016 | ||||||||||||||||||||
| (thousands of Canadian dollars) | $ | $ | $ | $ | ||||||||||||||||||||
| Cash flows used in operating activities: |
||||||||||||||||||||||||
| Net loss for the period |
(4,507) | (2,329) | (7,285) | (5,484) | ||||||||||||||||||||
| Adjustments: |
||||||||||||||||||||||||
| Amortization of intangible assets |
581 | 581 | 1,161 | 1,161 | ||||||||||||||||||||
| Depreciation of equipment |
86 | 34 | 173 | 62 | ||||||||||||||||||||
| Stock-based compensation |
9 | 295 | 210 | 331 | 275 | |||||||||||||||||||
| Net financial expenses (income) |
8 | 122 | (121) | 101 | 75 | |||||||||||||||||||
| Realized foreign exchange gain |
33 | 26 | 52 | 53 | ||||||||||||||||||||
| (3,390) | (1,599) | (5,467) | (3,858) | |||||||||||||||||||||
| Changes in non-cash operating items |
10 | 1,330 | 687 | 1,761 | 875 | |||||||||||||||||||
| Net cash used in operating activities |
(2,060) | (912) | (3,706) | (2,983) | ||||||||||||||||||||
| Cash flows from (used in) investing activities: |
||||||||||||||||||||||||
| Interest received |
14 | 11 | 30 | 22 | ||||||||||||||||||||
| Acquisition of equipment |
10 | (90) | (542) | (187) | (1,053) | |||||||||||||||||||
| Acquisition of short-term investments |
| (903) | | (9,266) | ||||||||||||||||||||
| Maturity of short-term investments |
| 3,834 | | 13,212 | ||||||||||||||||||||
| Net cash from (used in) investing activities |
(76) | 2,400 | (157) | 2,915 | ||||||||||||||||||||
| Cash flows used in financing activities: |
||||||||||||||||||||||||
| Payment of public offering transaction costs |
| | (381) | | ||||||||||||||||||||
| Payment of private placement transaction costs |
| | (40) | | ||||||||||||||||||||
| Interest paid |
(1) | (3) | (1) | (15) | ||||||||||||||||||||
| Net cash used in financing activities |
(1) | (3) | (422) | (15) | ||||||||||||||||||||
| Foreign exchange on cash and cash equivalents held in foreign currencies |
(101) | 17 | (158) | (51) | ||||||||||||||||||||
| Net (decrease) increase in cash and cash equivalents |
(2,238) | 1,502 | (4,443) | (134) | ||||||||||||||||||||
| Cash and cash equivalents, beginning of period |
7,567 | 1,391 | 9,772 | 3,027 | ||||||||||||||||||||
| Cash and cash equivalents, end of period |
5,329 | 2,893 | 5,329 | 2,893 | ||||||||||||||||||||
| Cash and cash equivalents is comprised of: |
||||||||||||||||||||||||
| Cash |
920 | 2,893 | 920 | 2,893 | ||||||||||||||||||||
| Cash equivalents |
4,409 | | 4,409 | | ||||||||||||||||||||
See accompanying notes to unaudited interim financial statements.
F-7
Table of Contents
ACASTI PHARMA INC.
Notes to Interim Financial Statements
(Unaudited)
Three-month and six-month periods ended September 30, 2017 and August 31, 2016
(thousands of Canadian dollars, except where noted and for share and per share amounts)
| 1. | Reporting entity: |
Acasti Pharma Inc. (Acasti or the Corporation) is incorporated under the Business Corporations Act (Québec) (formerly Part 1A of the Companies Act (Québec)). The Corporation is domiciled in Canada and its registered office is located at 545, Promenade du Centropolis, Laval, Québec, H7T 0A3. Neptune Technologies and Bioressources Inc. (Neptune or the parent) currently owns approximately 34% of the issued and outstanding Class A shares (Common Shares) of the Corporation. The Corporation, Neptune and Biodroga Nutraceuticals Inc., a subsidiary of Neptune, are collectively referred to as the Group.
Pursuant to a license agreement entered into with Neptune in August 2008, as amended, Acasti has been granted an exclusive worldwide license to use Neptunes intellectual property to develop, clinically study and market new pharmaceutical and medical food products to treat human cardiovascular conditions. Neptunes intellectual property is related to the extraction of ingredients from marine biomasses, such as krill. The eventual products are aimed at applications in the prescription drug, over-the-counter medicine and medical foods markets. In December 2012, the Corporation entered into a prepayment agreement with Neptune pursuant to which the Corporation exercised its option under the License Agreement to pay in advance all of the future royalties payable under the license which was exercised in fiscal 2014. As a result of the royalty prepayment, Acasti is no longer required to pay any royalties to Neptune under the License Agreement during its term for the use of the intellectual property under license. The license allows Acasti to exploit the intellectual property rights in order to develop novel active pharmaceutical ingredients (APIs) into commercial products for the prescription drugs and the medical food markets. On August 8, 2017, Neptune announced the sale of its krill oil inventory and intellectual property to Aker BioMarine Antarctic AS (Aker). Aker then licensed the intellectual property back to Neptune, leaving the License Agreement between Acasti and Neptune in place and unchanged. The license Agreement allows Acasti the freedom to operate for CaPre, which is currently the Corporations only prescription drug candidate in development. There are diligence obligations with respect to the Corporations use of licensed technology in relation to the development and commercialization of Acastis product candidate.
The Corporation is subject to a number of risks associated with the conduct of its clinical program and its results, the establishment of strategic alliances and the successful development of new pharmaceutical products and their marketing. The Corporation has incurred significant operating losses and negative cash flows from operations since inception. To date, the Corporation has financed its operations through the public offering and private placement of Common Shares and convertible debt, the proceeds from research grants and research tax credits, and the exercises of warrants, rights and options. To achieve the objectives of its business plan, Acasti plans to raise the necessary funds through additional securities offerings and the establishment of strategic alliances as well as additional research grants and research tax credits. The Corporation anticipates that the products developed by the Corporation will require approval from the U.S Food and Drug Administration and equivalent regulatory organizations in other countries before their sale can be authorized. The ability of the Corporation to ultimately achieve profitable operations is dependent on a number of factors outside of the Corporations control.
| 2. | Basis of preparation: |
| (a) | Statement of compliance: |
These interim financial statements have been prepared in accordance with International Accounting Standard (IAS) 34, Interim Financial Reporting, as issued by the International Accounting Standards Board (IASB) and on a basis consistent with those accounting policies followed by the Corporation and disclosed in note 3 of its most recent audited annual financial statements. Certain information, in particular the accompanying notes, normally included in the annual financial statements prepared in accordance with IFRS has been omitted or condensed. Accordingly, the condensed interim financial statements do not include all of the information required for full annual financial statements, and therefore, should be read in conjunction with the audited financial statements and the notes thereto for the year ended March 31, 2017.
Beginning in fiscal 2017, the Corporations fiscal year end is on March 31. As a result, the above financial statements and corresponding notes to financial statements include two different three-month and six-month periods: the three-month and six-month periods ended September 30, 2017 and the three-month and six-month periods ended August 31, 2016. Financial information for the three-month and six-month periods ended September 30, 2016 have not been included in these financial statements for the following reasons: (i) the three-month and six-month periods ended August 31, 2016 provide a meaningful comparison for the three-month and six-month periods ended September 30, 2017; (ii) there are no significant factors, seasonal or otherwise, that would impact the comparability of information if the results for the three-month and six-month periods ended September 30, 2016 were presented in lieu of results for the three-month and six-month periods ended August 31, 2016; and (iii) it was not practicable or cost justified to prepare this information.
The financial statements were authorized for issue by the Board of Directors on November 13, 2017.
F-8
Table of Contents
ACASTI PHARMA INC.
Notes to Interim Financial Statements
(Unaudited)
Three-month and six-month periods ended September 30, 2017 and August 31, 2016
(thousands of Canadian dollars, except where noted and for share and per share amounts)
| 2. | Basis of preparation (continued): |
| (b) | Basis of measurement: |
The financial statements have been prepared on the historical cost basis, except for:
| | Stock-based compensation which is measured pursuant to IFRS 2, Share-based payments (note 9); and, |
| | Derivative warrant liabilities measured at fair value on a recurring basis (note 5). |
| (c) | Going concern uncertainty: |
The Corporation has incurred operating losses and negative cash flows from operations since inception. The Corporations current assets of $5.9 million as at September 30, 2017 include cash and cash equivalents totalling $5.3 million, mainly generated by the net proceeds from the Public Offering and Private Placement completed on February 21, 2017. The Corporations liabilities total $5.0 million at September 30, 2017 and are comprised primarily of $3.4 million in amounts due to or accrued for creditors and $1.5 million for unsecured convertible debentures. The Corporations positive working capital balance has declined since the Previous Offerings and is expected to continue to decline until the Corporation raises additional funds or finds a strategic partner. The Corporations current assets as at this date are projected to be significantly less than needed to support the current liabilities as at that date when combined with the projected level of expenses for the next twelve months, including not only the preparation for, but the planned site activation of and patient treatment within the Phase 3 clinical study program for its drug candidate, CaPre. Additional funds will also be needed for the expected expenses for the total CaPre Phase 3 research and development phase beyond the next twelve months. The Corporation is working towards development of strategic partner relationships and plans to raise additional funds in the near future, but there can be no assurance as to when or whether Acasti will complete any financing or strategic collaborations. In particular, raising financing is subject to market conditions and is not within the Corporations control. If the Corporation does not raise additional funds or find one or more strategic partners, it may not be able to realize its assets and discharge its liabilities in the normal course of business. As a result, there exists a material uncertainty that casts substantial doubt about the Corporations ability to continue as a going concern and, therefore, realize its assets and discharge its liabilities in the normal course of business. The Corporation currently has no other arranged sources of financing.
The financial statements have been prepared on a going concern basis, which assumes the Corporation will continue its operations in the foreseeable future and will be able to realize its assets and discharge its liabilities and commitments in the ordinary course of business. These financial statements do not include any adjustments to the carrying values and classification of assets and liabilities and reported expenses that may be necessary if the going concern basis was not appropriate for these financial statements. If the Corporation was unable to continue as a going concern, material write-downs to the carrying values of the Corporations assets, including the intangible asset, could be required.
| (d) | Functional and presentation currency: |
These financial statements are presented in Canadian dollars, which is the Corporations functional currency.
| (e) | Use of estimates and judgments: |
The preparation of the financial statements in conformity with IFRS requires management to make judgments, estimates and assumptions that affect the application of accounting policies and the reported amounts of assets, liabilities, income and expenses. Actual results may differ from these estimates.
Estimates are based on managements best knowledge of current events and actions that the Corporation may undertake in the future. Estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimates are revised and in any future periods affected.
F-9
Table of Contents
ACASTI PHARMA INC.
Notes to Interim Financial Statements
(Unaudited)
Three-month and six-month periods ended September 30, 2017 and August 31, 2016
(thousands of Canadian dollars, except where noted and for share and per share amounts)
| 2. | Basis of preparation (continued): |
| (e) | Use of estimates and judgments (continued): |
Critical judgments in applying accounting policies that have the most significant effect on the amounts recognized in the financial statements include the following:
| | Identification of triggering events indicating that the intangible assets might be impaired. |
| | The use of the going concern basis of preparation of the financial statements. At the end of each reporting period, management assesses the basis of preparation of the financial statements (Note 2(c)). |
Assumptions and estimation uncertainties that have a significant risk of resulting in a material adjustment within the next financial year include the following:
| | Determination of the recoverable amount of the Corporations cash generating unit (CGU). |
| | Measurement of derivative warrant liabilities (note 5) and share-based payments (note 9). |
Also, management uses judgment to determine which research and development (R&D) expenses qualify for R&D tax credits and in what amounts. The Corporation recognizes the tax credits once it has reasonable assurance that they will be realized. Recorded tax credits are subject to review and approval by tax authorities and therefore, could be different from the amounts recorded.
| 3. | Significant accounting policies: |
The accounting policies and basis of measurement applied in these interim financial statements are the same as those applied by the Corporation in its financial statements for the year ended March 31, 2017.
New standards and interpretations not yet adopted:
| (i) | Financial instruments: |
On July 24, 2014, the International Accounting Standards Board (IASB) issued the final version of IFRS 9, Financial Instruments, which addresses the classification and measurement of financial assets and liabilities, impairment and hedge accounting, replacing IAS 39, Financial Instruments: Recognition and Measurement. IFRS 9 is effective for annual periods beginning on or after January 1, 2018, with earlier adoption permitted. The Corporation intends to adopt IFRS 9 in its financial statements for the annual period beginning on April 1, 2018. The Corporation has not yet assessed the impact of adoption of IFRS 9, and does not intend to early adopt IFRS 9 in its financial statements.
| (ii) | Amendments to IFRS 2 Classification and Measurement of Share-Based Payment Transactions: |
On June 20, 2016, the IASB issued amendments to IFRS 2, Share-Based Payment, clarifying how to account for certain types of share-based payment transactions. The amendments apply for annual periods beginning on or after January 1, 2018. Earlier application is permitted. As a practical simplification, the amendments can be applied prospectively. Retrospective, or early application is permitted if information is available without the use of hindsight. The amendments provide requirements on the accounting for: the effects of vesting and non-vesting conditions on the measurement of cash-settled share-based payments; share-based payment transactions with a net settlement feature for withholding tax obligations; and a modification to the terms and conditions of a share-based payment that changes the classification of the transaction from cash-settled to equity-settled. The Corporation intends to adopt the amendments to IFRS 2 in its financial statements for the annual period beginning on April 1, 2018. The Corporation has not yet assessed the impact of adoption of the amendments of IFRS 2, and does not intend to early adopt these amendments in its financial statements.
F-10
Table of Contents
ACASTI PHARMA INC.
Notes to Interim Financial Statements
(Unaudited)
Three-month and six-month periods ended September 30, 2017 and August 31, 2016
(thousands of Canadian dollars, except where noted and for share and per share amounts)
| 4. | Related parties: |
| (a) | Administrative and research and development expenses: |
The Corporation intends to continue to rely on the support of Neptune for a portion of its general and administrative needs; however, the continuance of this support is outside of the Corporations control.
During the three-month and six-month periods ended September 30, 2017 and August 31, 2016, the Corporation was charged by Neptune for the purchase of research supplies, for certain costs incurred by Neptune for the benefit of the Corporation and for a shared service agreement as follows:
| Three-month periods ended | Six-month periods ended | |||||||||||||||
| September 30, 2017 | August 31, 2016 | September 30, 2017 | August 31, 2016 | |||||||||||||
| $ | $ | $ | $ | |||||||||||||
| Research and development expenses |
||||||||||||||||
| Supplies and incremental costs |
| | 6 | | ||||||||||||
| Shared service agreement |
8 | 9 | 20 | 9 | ||||||||||||
| 8 | 9 | 26 | 9 | |||||||||||||
| General and administrative expenses |
||||||||||||||||
| Supplies and incremental costs |
56 | 57 | 109 | 108 | ||||||||||||
| Shared service agreement |
37 | 75 | 87 | 150 | ||||||||||||
| 93 | 132 | 196 | 258 | |||||||||||||
| 101 | 141 | 222 | 267 | |||||||||||||
Where Neptune incurs specific incremental costs for the benefit of the Corporation, it charges those amounts directly. Costs that benefit more than one entity of the Group are charged by allocating a fraction of costs incurred by Neptune that is commensurate to the estimated fraction of services or benefits received by each entity for those items. In addition, Neptune provides Acasti with the services of personnel for its administrative, legal and laboratory work as part of a shared service agreement. The employees salaries and benefits are charged proportionally to the time allocation agreed upon within the shared service agreement. In the three-month period ended September 30, 2017, the laboratory support, the corporate affairs and the public company reporting services previously provided by Neptune as part of the shared service agreement were discontinued. The Corporation is now incurring some incremental costs and expects to do so in the future, partially offset by reduced shared service fees.
Historically, Neptune has provided the Corporation with the krill oil needed to produce CaPre for Acastis clinical programs, including all of the krill oil projected as needed for its Phase 3 clinical study program. However, in light of Neptunes recent announcement of its plan to discontinue krill oil production and the sale of its krill oil inventory to Aker, the Corporation is evaluating alternative suppliers of krill oil.
These charges do not represent all charges incurred by Neptune that may have benefited the Corporation. Also, these charges do not necessarily represent the cost that the Corporation would otherwise need to incur, should it not receive these services or benefits through the shared resources of Neptune.
The Corporation purchased from the parent company research and development supplies of which $25 as at September 30, 2017 is recorded in prepaid expenses and will be expensed as used.
F-11
Table of Contents
ACASTI PHARMA INC.
Notes to Interim Financial Statements
(Unaudited)
Three-month and six-month periods ended September 30, 2017 and August 31, 2016
(thousands of Canadian dollars, except where noted and for share and per share amounts)
| 4. | Related parties (continued): |
| (b) | Interest revenue: |
On January 7, 2016 Neptune announced the acquisition of Biodroga Nutraceuticals Inc. As part of this transaction, the Corporation pledged an amount of $2 million (Committed Funds) to partly guarantee the financing of this transaction (Pledge Agreement). Neptune had agreed to pay Acasti an annual fee on the Committed Funds outstanding at an annual rate of 9% during the first six months and 11% for the remaining term of the Pledge Agreement. On September 20, 2016, Neptune fully released the pledged amount. The Corporation recognized interest revenue related to this arrangement in the amount of nil for the three-month and six-month periods ended September 30, 2017 and $38 and $83 for the three- month and six-month periods ended August 31, 2016.
| (c) | Payable to parent corporation: |
Payable to parent corporation, primarily for general and administrative shared services, has no specified maturity date for payment or reimbursement and does not bear interest.
| (d) | Key management personnel compensation: |
The key management personnel are the officers of the Corporation, the members of the Board of Directors of the Corporation and of the parent company. They control in the aggregate less than 2% of the voting shares of the Corporation (2% at August 31, 2016).
Key management personnel compensation includes the following for the three-month and six-month periods ended September 30, 2017 and August 31, 2016:
| Three-month periods ended | Six-month periods ended | |||||||||||||||
| September 30, 2017 | August 31, 2016 | September 30, 2017 | August 31, 2016 | |||||||||||||
| $ | $ | $ | $ | |||||||||||||
| Short-term salaries and benefits |
345 | 286 | 705 | 557 | ||||||||||||
| Share-based compensation costs |
264 | 198 | 286 | 244 | ||||||||||||
| 609 | 484 | 991 | 801 | |||||||||||||
F-12
Table of Contents
ACASTI PHARMA INC.
Notes to Interim Financial Statements
(Unaudited)
Three-month and six-month periods ended September 30, 2017 and August 31, 2016
(thousands of Canadian dollars, except where noted and for share and per share amounts)
| 5. | Derivative warrant liabilities: |
Warrants issued as part of a public offering of units composed of class A share (Common Share) and Common Share purchase warrants in 2014 are derivative liabilities (Derivative warrant liabilities) given the currency of the exercise price is different from the Corporations functional currency.
The derivative warrant liabilities are measured at fair value at every reporting period and the reconciliation of changes in fair value for the six-month periods ended September 30, 2017 and August 31, 2016 is presented in the following table:
| Six-month periods ended | ||||||
|
|
||||||
| September 30, 2017 | August 31, 2016 | |||||
| $ | $ | |||||
| Balance beginning of period |
209 | 156 | ||||
| Change in fair value of derivative warrant liabilities |
(158) | (98) | ||||
| Balance end of period |
51 | 58 | ||||
The fair value of the derivative warrant liabilities was estimated using the Black-Scholes option pricing model and based on the following assumptions:
| September 30, 2017 | March 31, 2017 | |||||||||||
| Exercise price |
US $1.50 | US $1.50 | ||||||||||
| Share price(1) |
US $1.42 | US $1.36 | ||||||||||
| Risk-free interest |
1.35% | 1.22% | ||||||||||
| Estimated life |
1.18 years | 1.68 years | ||||||||||
| Expected volatility |
100.5% | 108.4% | ||||||||||
| (1) | In order to obtain one Common Share, 10 warrants must be exercised. |
The fair value of the warrants issued was determined to be $0.03 per share issuable as at September 30, 2017 (0.11 per share issuable as at March 31, 2017).
| 6. | Capital and other components of equity: |
| (a) | Issuance of shares: |
The following table summarizes the shares issued to settle the payment of accrued interest on the unsecured convertible debentures with the corresponding amount recorded to share capital.
| Accrued interest as at | Share issuance date | Number of shares | Amount | |||
| $ | ||||||
| March 31, 2017 |
April 7, 2017 | 9,496 | 17 | |||
| June 30, 2017 |
August 15, 2017 | 23,885 | 40 | |||
| 33,381 | 57 | |||||
F-13
Table of Contents
ACASTI PHARMA INC.
Notes to Interim Financial Statements
(Unaudited)
Three-month and six-month periods ended September 30, 2017 and August 31, 2016
(thousands of Canadian dollars, except where noted and for share and per share amounts)
| 6. | Capital and other components of equity (continued): |
| (b) | Warrants: |
The warrants of the Corporation are composed of the following as at September 30, 2017 and March 31, 2017:
| September 30, 2017 | March 31, 2017 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Number outstanding |
Amount | Number outstanding |
Amount | |||||||||||||||||
| $ | $ | |||||||||||||||||||
| Liability |
||||||||||||||||||||
| Series 8 Public offering |
||||||||||||||||||||
| Warrants 2014 (i) |
18,400,000 | 51 | 18,400,000 | 209 | ||||||||||||||||
| 18,400,000 | 51 | 18,400,000 | 209 | |||||||||||||||||
| Equity |
||||||||||||||||||||
| Public offering warrants |
||||||||||||||||||||
| Public offering warrants 2017 (ii) |
1,965,259 | | 1,965,259 | | ||||||||||||||||
| Series 2017-BW Broker warrants (iii) |
234,992 | 144 | 234,992 | 144 | ||||||||||||||||
| Private Placement contingent warrants |
||||||||||||||||||||
| 2017 Unsecured convertible debenture conversion option and contingent warrants (iv) |
1,052,630 | 309 | 1,052,630 | 309 | ||||||||||||||||
| Series 9 Private Placement warrants 2014 (v) |
161,654 | | 161,654 | | ||||||||||||||||
| 3,414,535 | 453 | 3,414,535 | 453 | |||||||||||||||||
| (i) | In order to obtain one Common Share of the Corporation at an exercise price of US$15.00, 10 warrants must be exercised. Warrants expire on December 3, 2018. |
| (ii) | Warrant to acquire one Common Share of the Corporation at an exercise price of $2.15, expiring on February 21, 2022. |
| (iii) | Warrant to acquire one Common Share of the Corporation at an exercise price of $2.15 expiring on February 21, 2018. |
| (iv) | Warrant to acquire one Common Share of the Corporation at an exercise price of $1.90 expiring on February 21, 2020, net of deferred tax expense of $129. |
| (v) | Warrant to acquire one Common Share of the Corporation at an exercise price of $13.30, expiring on December 3, 2018. |
| 7. | Government assistance: |
|
|
||||||||||||||||||||
| Three-month periods ended | Six-month periods ended | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| September 30, 2017 | August 31, 2016 | September 30, 2017 | August 31, 2016 | |||||||||||||||||
|
|
||||||||||||||||||||
| $ | $ | $ | $ | |||||||||||||||||
|
|
||||||||||||||||||||
| Investment tax credit |
37 | 23 | 59 | 47 | ||||||||||||||||
| Government grant |
1 | 12 | 1 | 47 | ||||||||||||||||
|
|
||||||||||||||||||||
| 38 | 35 | 60 | 94 | |||||||||||||||||
|
|
||||||||||||||||||||
F-14
Table of Contents
ACASTI PHARMA INC.
Notes to Interim Financial Statements
(Unaudited)
Three-month and six-month periods ended September 30, 2017 and August 31, 2016
(thousands of Canadian dollars, except where noted and for share and per share amounts)
8. Financial (expenses) income:
| Three-month periods ended | Six-month periods ended | |||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| September 30, 2017 | August 31, 2016 | September 30, 2017 | August 31, 2016 | |||||||||||||||||
| $ | $ | $ | $ | |||||||||||||||||
| Financial income Interest |
14 | 47 | 30 | 106 | ||||||||||||||||
| Foreign exchange (loss) gain |
(68) | 10 | (104) | (264) | ||||||||||||||||
| Interest payable on convertible debenture |
(41) | | (80) | | ||||||||||||||||
| Accretion of interest on convertible debenture |
(51) | | (103) | | ||||||||||||||||
| Other charges |
| (2) | (2) | (15) | ||||||||||||||||
| Financial (expenses) income |
(160) | 8 | (289) | (279) | ||||||||||||||||
| Net financial (expenses) income |
(146) | 55 | (259) | (173) | ||||||||||||||||
|
|
||||||||||||||||||||
| 9. | Share-based payment: |
At September 30, 2017 the Corporation has the following share-based payment arrangements:
Corporation stock option plan:
The Corporation has in place a stock option plan for directors, officers, employees and consultants of the Corporation (Stock Option Plan). The plan provides for the granting of options to purchase Class A shares (Common Shares). The exercise price of the stock options granted under this plan is not lower than the closing price of the shares listed on the TSXV at the close of markets the day preceding the grant. Under this plan, the maximum number of Class A shares (Common Shares) that may be issued upon exercise of options granted under the plan is 2,940,511, representing 20% of the number of Class A shares (Common Shares) issued and outstanding as at March 31, 2017. The terms and conditions for acquiring and exercising options are set by the Corporations Board of Directors, subject among others, to the following limitations: the term of the options cannot exceed ten years and every stock option granted under the stock option plan will be subject to conditions no less restrictive than a minimum vesting period of 18 months and a gradual and equal acquisition of vesting rights not shorter than on a quarterly basis. The total number of shares issued to any one consultant cannot exceed 2% of the Corporations total issued and outstanding shares. The Corporation is not authorized to grant such number of options under the stock option plan that could result in a number of Class A shares (Common Shares) issuable pursuant to options granted to (a) related persons exceeding 10% of the Corporations issued and outstanding Class A shares (Common Shares) (on a non-diluted basis) on the date an option is granted, or (b) any one eligible person in a twelve month period exceeding 5% of the Corporations issued and outstanding Class A shares (Common Shares) (on a non-diluted basis) on the date an option is granted.
The following table summarizes information about activities within the stock option plan for the six-month periods ended:
|
|
||||||||||||||||||||
| September 30, 2017 | August 31, 2016 | |||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Weighted average exercise price |
Number of options |
Weighted average exercise price |
Number of options |
|||||||||||||||||
|
|
||||||||||||||||||||
| $ | $ | |||||||||||||||||||
|
|
||||||||||||||||||||
| Outstanding at beginning of period |
2.58 | 1,424,788 | 13.52 | 454,151 | ||||||||||||||||
| Granted |
1.75 | 1,121,500 | 1.72 | 835,400 | ||||||||||||||||
| Forfeited |
2.17 | (92,600) | 14.13 | (128,750) | ||||||||||||||||
| Expired |
20.82 | (51,500) | 14.65 | (123,000) | ||||||||||||||||
|
|
||||||||||||||||||||
| Outstanding at end of period |
1.82 | 2,402,188 | 3.81 | 1,037,801 | ||||||||||||||||
|
|
||||||||||||||||||||
| Exercisable at end of period |
2.16 | 394,346 | 11.92 | 197,845 | ||||||||||||||||
|
|
||||||||||||||||||||
F-15
Table of Contents
ACASTI PHARMA INC.
Notes to Interim Financial Statements
(Unaudited)
Three-month and six-month periods ended September 30, 2017 and August 31, 2016
(thousands of Canadian dollars, except where noted and for share and per share amounts)
| 9. | Share-based payment (continued): |
Corporation stock option plan (continued):
The fair value of options granted has been estimated according to the Black-Scholes option pricing model and based on the weighted average of the following assumptions for options granted during the six-month periods ended:
| September 30, 2017 | August 31, 2016 | |||||||
| Exercise price |
$1.75 | $1.72 | ||||||
| Share price |
$1.75 | $1.72 | ||||||
| Risk-free interest |
1.21% | 0.70% | ||||||
| Estimated life |
5.89 years | 4.38 years | ||||||
| Expected volatility |
82.4% | 75.5% | ||||||
The weighted average fair value of the options granted to employees and directors during the six-month period ended September 30, 2017 was $1.22 (six-month period ended August 31, 2016 - $0.99) and no options were granted to consultants. For the three-month and six-month periods ended September 30, 2017, the Corporation recognized stock-based compensation under this plan in the amount of $295 and $331, respectively (three-month and six-months periods ended August 31, 2016 - $210 and $275 respectively).
Share-based payment transactions and broker warrants:
The fair value of share-based payment transaction is measured using the Black-Scholes valuation model. Measurement inputs include share price on measurement date, exercise price of the instrument, expected volatility (based on weighted average historic volatility), weighted average expected life of the instruments (based on historical experience and general option holder behaviour unless no entity-specific information exists in which case the average of the vesting and contractual periods is used), expected dividends, and the risk-free interest rate (based on government bonds). Service and non-market performance conditions attached to the transactions, if any, are not taken into account in determining fair value.
| 10. | Supplemental cash flow disclosure: |
| (a) | Changes in non-cash operating items: |
|
|
||||||||||||||||||||
| Three-month periods ended | Six-month periods ended | |||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| September 30, 2017 | August 31, 2016 | September 30, 2017 | August 31, 2016 | |||||||||||||||||
|
|
||||||||||||||||||||
| $ | $ | $ | $ | |||||||||||||||||
|
|
||||||||||||||||||||
| Receivables |
(133) | 56 | (37) | 230 | ||||||||||||||||
| Prepaid expenses |
26 | 590 | 23 | 220 | ||||||||||||||||
| Trade and other payables |
1,445 | (121) | 1,719 | 235 | ||||||||||||||||
| Receivable from/payable to parent corporation |
(8) | 162 | 56 | 190 | ||||||||||||||||
|
|
||||||||||||||||||||
| 1,330 | 687 | 1,761 | 875 | |||||||||||||||||
|
|
||||||||||||||||||||
F-16
Table of Contents
ACASTI PHARMA INC.
Notes to Interim Financial Statements
(Unaudited)
Three-month and six-month periods ended September 30, 2017 and August 31, 2016
(thousands of Canadian dollars, except where noted and for share and per share amounts)
| 10. | Supplemental cash flow disclosure (continued): |
| (b) | Non-cash transactions: |
|
|
||||||||||||||||||||
| Three-month periods ended | Six-month periods ended | |||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| September 30, 2017 | August 31, 2016 | September 30, 2017 | August 31, 2016 | |||||||||||||||||
|
|
||||||||||||||||||||
| $ | $ | $ | $ | |||||||||||||||||
|
|
||||||||||||||||||||
| Equipment included in trade and other Payables |
165 | | 165 | | ||||||||||||||||
|
Interest payable included in trade and other payables |
40 | | 40 | | ||||||||||||||||
|
Interest receivable included in payable to Parent corporation |
| 83 | | 83 | ||||||||||||||||
|
|
||||||||||||||||||||
| 11. | Commitments and contingencies: |
Research and development agreements:
In the normal course of business, the Corporation has signed agreements with various suppliers for them to execute research and development projects and to produce certain tools and equipment. The Corporation has reserved certain rights relating to these projects.
The Corporation initiated research and development projects that are planned to be conducted over the next 12-month period. As at September 30, 2017, of these research and development agreements, an amount of $1,608 is included in Trade and other payables and an amount of $2,786 remains a future commitment.
The Corporation has also entered into a contract to purchase production equipment to be used in the manufacturing of the clinical and future commercial supply of CaPre®. As at September 30, 2017, of this equipment, an amount of $165 is included in Trade and other payables and an amount of $283 remains a future commitment.
Contingencies:
A former CEO of the Corporation is claiming the payment of approximately $8.5 million and the issuance of equity instruments from the Group. As the Corporations management believes that these claims are not valid, no provision has been recognized. Neptune and its subsidiaries also filed an additional claim to recover certain amounts from the former officer. All outstanding share-based payments held by the former CEO have been cancelled during the year ended February 28, 2015.
F-17
Table of Contents
ACASTI PHARMA INC.
Notes to Interim Financial Statements
(Unaudited)
Three-month and six-month periods ended September 30, 2017 and August 31, 2016
(thousands of Canadian dollars, except where noted and for share and per share amounts)
| 11. | Commitments and contingencies (continued): |
Contingencies: (continued):
The Corporation is also involved in other matters arising in the ordinary course of its business. Since management believes that all related claims are not valid and it is presently not possible to determine the outcome of these matters, no provisions have been made in the financial statements for their ultimate resolution beyond the amounts incurred and recorded for such matters. The resolution of such matters could have an effect on the Corporations financial statements in the year that a determination is made, however, in managements opinion, the final resolution of all such matters is not projected to have a material adverse effect on the Corporations financial position.
| 12. | Determination of fair values: |
Certain of the Corporations accounting policies and disclosures require the determination of fair value, for both financial assets and liabilities. Fair values have been determined for measurement and/or disclosure purposes based on the following methods.
Financial assets and liabilities:
In establishing fair value, the Corporation uses a fair value hierarchy based on levels as defined below:
| | Level 1: defined as observable inputs such as quoted prices in active markets. |
| | Level 2: defined as inputs other than quoted prices in active markets that are either directly or indirectly observable. |
| | Level 3: defined as inputs that are based on little or no observable market data, therefore requiring entities to develop their own assumptions. |
The Corporation has determined that the carrying values of its short-term financial assets and liabilities approximate their fair value given the short-term nature of these instruments. The fair value of the liability component of the convertible debenture is determined by discounting future cash flows using a rate that the Corporation could obtain for loans with similar terms, conditions and maturity dates. The fair value of this liability at September 30, 2017 approximates the carrying amount and was measured using level 3 inputs.
Derivative warrant liabilities:
The Corporation measured its derivative warrant liabilities at fair value on a recurring basis. These financial liabilities were measured using a level 3 inputs (note 5).
As at September 30, 2017, the effect of an increase or a decrease of 5% of the volatility used, which is the significant unobservable input in the fair value estimate, would result in a loss of $19 or a gain of $15, respectively.
F-18
Table of Contents
Financial Statements of
ACASTI PHARMA INC.
For the thirteen-month and one-month periods ended March 31, 2017, twelve-month period ended February 28, 2017 and years ended February 29, 2016 and February 28, 2015
F-19
Table of Contents
INDEPENDENT AUDITORS REPORT OF REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders of Acasti Pharma Inc.
We have audited the accompanying financial statements of Acasti Pharma Inc., which comprise the statements of financial position as at March 31, 2017 and February 29, 2016, the statements of earnings and comprehensive loss, changes in equity and cash flows for the thirteen-month period ended March 31, 2017 and the years ended February 29, 2016 and February 28, 2015, and notes, comprising a summary of significant accounting policies and other explanatory information.
Managements Responsibility for the Financial Statements
Management is responsible for the preparation and fair presentation of these financial statements in accordance with International Financial Reporting Standards as issued by the International Accounting Standards Board, and for such internal control as management determines is necessary to enable the preparation of financial statements that are free from material misstatement, whether due to fraud or error.
Auditors Responsibility
Our responsibility is to express an opinion on these financial statements based on our audits. We conducted our audits in accordance with Canadian generally accepted auditing standards and the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we comply with ethical requirements and plan and perform the audit to obtain reasonable assurance about whether the financial statements are free from material misstatement.
An audit involves performing procedures to obtain audit evidence about the amounts and disclosures in the financial statements. The procedures selected depend on our judgment, including the assessment of the risks of material misstatement of the financial statements, whether due to fraud or error. In making those risk assessments, we consider internal control relevant to the entitys preparation and fair presentation of the financial statements in order to design audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the entitys internal control. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, evaluating the appropriateness of accounting policies used and the reasonableness of accounting estimates made by management, as well as evaluating the overall presentation of the financial statements.
We believe that the audit evidence we have obtained in our audits is sufficient and appropriate to provide a basis for our audit opinion.
Opinion
In our opinion, the financial statements present fairly, in all material respects, the financial position of Acasti Pharma Inc. as at March 31, 2017 and February 29, 2016, and its financial performance and its cash flows for the thirteen-month period ended March 31, 2017 and years ended February 29, 2016 and February 28, 2015 in accordance with International Financial Reporting Standards as issued by the International Accounting Standards Board.
Other matter
The financial statements of Acasti Pharma Inc. as at February 28, 2017 and for the twelve-month and one-month periods ended February 28, 2017 and March 31, 2017 respectively are unaudited. Accordingly, we do not express an opinion on them.
Emphasis of matter
Without qualifying our opinion, we draw attention to Note 2(c) in the financial statements which indicates that Acasti Pharma Inc. has incurred operating losses and negative cash flows from operations since inception, that the Corporations current assets as at March 31, 2017 are projected to be significantly less than needed and that its future operations are dependent on obtaining additional financing and on the continued support of its parent corporation for a portion of its general and administrative needs. These conditions, along with other matters as set forth in 2(c) in the financial statements, indicate the existence of a material uncertainty that casts substantial doubt about Acasti Pharma Inc.s ability to continue as a going concern.
/s/ KPMG LLP*
June 6, 2017
| Montréal, Canada | ||
| *CPA auditor, CA, public accountancy permit No. A119178 | ||
| KPMG LLP is a Canadian limited liability partnership and a member firm of the KPMG | ||
| network of independent member firms affiliated with KPMG International Cooperative | ||
| (KPMG International), a Swiss entity. | ||
| KPMG Canada provides services to KPMG LLP. |
F-20
Table of Contents
ACASTI PHARMA INC.
Financial Statements
Thirteen-month and one-month periods ended March 31, 2017, twelve-month period ended February 28, 2017 and years ended February 29, 2016 and February 28, 2015
Financial Statements
| F-22 | ||||
| F-23 | ||||
| F-24 | ||||
| F-26 | ||||
| F-27 | ||||
F-21
Table of Contents
Statements of Financial Position
March 31, 2017, February 28, 2017 and February 29, 2016
| Notes | March 31, 2017 |
February 28, 2017 (Unaudited) |
February 29, 2016 | |||||||||||||
| (thousands of Canadian dollars) | $ | $ | $ | |||||||||||||
| Assets |
||||||||||||||||
| Current assets: |
||||||||||||||||
| Cash and cash equivalents |
22 | 9,772 | 10,573 | 3,027 | ||||||||||||
| Short-term investments |
| | 7,443 | |||||||||||||
| Receivables |
4 | 206 | 166 | 399 | ||||||||||||
| Prepaid expenses |
209 | 176 | 456 | |||||||||||||
| 10,187 | 10,915 | 11,325 | ||||||||||||||
| Restricted short-term investment |
5(b) | | | 2,000 | ||||||||||||
| Equipment |
7 | 2,881 | 2,870 | 287 | ||||||||||||
| Intangible assets |
8 | 12,388 | 12,582 | 14,905 | ||||||||||||
| Total assets |
25,456 | 26,367 | 28,517 | |||||||||||||
| Liabilities and Equity |
||||||||||||||||
| Current liabilities: |
||||||||||||||||
| Trade and other payables |
9 | 2,126 | 2,390 | 1,126 | ||||||||||||
| Payable to parent corporation |
5(c) | 12 | 15 | 15 | ||||||||||||
| 2,138 | 2,405 | 1,141 | ||||||||||||||
| Derivative warrant liabilities |
10, 12(d) | 209 | 187 | 156 | ||||||||||||
| Unsecured convertible debentures |
11 | 1,406 | 1,389 | | ||||||||||||
| Total liabilities |
3,753 | 3,981 | 1,297 | |||||||||||||
| Equity: |
||||||||||||||||
| Share capital |
12 | 66,576 | 66,576 | 61,973 | ||||||||||||
| Other equity |
11 | 309 | 309 | | ||||||||||||
| Contributed surplus |
5,693 | 5,607 | 4,875 | |||||||||||||
| Deficit |
(50,875) | (50,106) | (39,628) | |||||||||||||
| Total equity |
21,703 | 22,386 | 27,220 | |||||||||||||
| Commitments and contingencies |
20 | |||||||||||||||
| Total liabilities and equity |
25,456 | 26,367 | 28,517 | |||||||||||||
See accompanying notes to financial statements.
On behalf of the Board:
| /s/ Dr. Roderick Carter | /s/ Jean-Marie Canan | |||
| Roderick Carter | Jean-Marie Canan | |||
| Chair of the Board | Director |
F-22
Table of Contents
Statements of Earnings and Comprehensive Loss
Thirteen-month and one-month periods ended March 31, 2017, twelve-month period ended February 28, 2017 and years ended February 29, 2016 and February 28, 2015
|
Thirteen-months ended |
Month ended |
Twelve-months ended |
Year ended | Year ended | ||||||||||||||||||||
| Notes | March 31, 2017 |
March 31, 2017 |
February 28, 2017 |
February 29, 2016 |
February 28, 2015 |
|||||||||||||||||||
| (Unaudited) | (Unaudited) | |||||||||||||||||||||||
| (thousands of Canadian dollars, except per share data) | $ | $ | $ | $ | $ | |||||||||||||||||||
| Research and development expenses, net of government assistance of $330 (March 2017 - $45 (unaudited); February 2017- $285 (unaudited), 2016 - $349, 2015 - $264) |
(7,653) | (426) | (7,227) | (7,566) | (8,822) | |||||||||||||||||||
| General and administrative expenses |
(3,557) | (292) | (3,265) | (2,046) | (3,573) | |||||||||||||||||||
| Loss from operating activities |
(11,210) | (718) | (10,492) | (9,612) | (12,395) | |||||||||||||||||||
| Financial (expenses) income |
14 | (113) | (29) | (84) | 1,094 | 1,916 | ||||||||||||||||||
| Change in fair value of warrant liabilities |
10 | (53) | (22) | (31) | 2,201 | 8,824 | ||||||||||||||||||
| Net financial (expenses) income |
(166) | (51) | (115) | 3,295 | 10,740 | |||||||||||||||||||
| Net loss and comprehensive loss before income tax |
(11,376) | (769) | (10,607) | (6,317) | (1,655) | |||||||||||||||||||
| Deferred income tax recovery |
129 | | 129 | | | |||||||||||||||||||
| Net loss and total comprehensive loss |
(11,247) | (769) | (10,478) | (6,317) | (1,655) | |||||||||||||||||||
| Basic and diluted loss per share |
16 | (1.01) | (0.05) | (0.97) | (0.59) | (0.16) | ||||||||||||||||||
| Weighted average number of shares outstanding |
11,094,512 | 14,702,556 | 10,788,075 | 10,659,936 | 10,617,704 | |||||||||||||||||||
See accompanying notes to financial statements
F-23
Table of Contents
Statements of Changes in Equity
Thirteen-month and one-month periods ended March 31, 2017, twelve-month period ended February 28, 2017 and years ended February 29, 2016 and February 28, 2015
| Share capital | Other | Contributed | ||||||||||||||||||||||||
| Notes | Number | Dollar | equity | surplus | Deficit | Total | ||||||||||||||||||||
| (thousands of Canadian dollars) | $ | $ | $ | $ | $ | |||||||||||||||||||||
| Balance, February 29, 2016 |
10,712,038 | 61,973 | | 4,875 | (39,628) | 27,220 | ||||||||||||||||||||
| Net loss and total comprehensive loss for the twelve-month period (unaudited) |
| | | | (10,478) | (10,478) | ||||||||||||||||||||
| Net loss and total comprehensive loss for the one-month period (unaudited) |
| | | | (769) | (769) | ||||||||||||||||||||
| Net loss and total comprehensive loss for the thirteen-month period |
| | | | (11,247) | (11,247) | ||||||||||||||||||||
| 10,712,038 | 61,973 | | 4,875 | (50,875) | 15,973 | |||||||||||||||||||||
| Transactions with owners, recorded directly in equity |
||||||||||||||||||||||||||
| Contributions by and distributions to equity holders |
||||||||||||||||||||||||||
| Public offering |
12(b) | 3,930,518 | 4,509 | | 144 | | 4,653 | |||||||||||||||||||
| Issue of unsecured convertible debentures, net of deferred income tax expense of $129 |
11,18 | | | 309 | | | 309 | |||||||||||||||||||
| Equity settled non-employee share-based payment |
12(b) | 60,000 | 94 | | | | 94 | |||||||||||||||||||
| Share-based payment transactions for the twelve-month period (unaudited) |
15 | | | | 588 | | 588 | |||||||||||||||||||
| Share-based payment transactions for the one-month period (unaudited) |
15 | | | | 86 | | 86 | |||||||||||||||||||
| Share-based payment transactions for the thirteen-month period |
15 | | | | 674 | | 674 | |||||||||||||||||||
| Total contributions by and distributions to equity holders for the twelve-month period (unaudited) |
3,990,518 | 4,603 | 309 | 732 | | 5,644 | ||||||||||||||||||||
| Total contributions by and distributions to equity holders for the one-month period (unaudited) |
| | | 86 | | 86 | ||||||||||||||||||||
| Total contributions by and distributions to equity holders for the thirteen-month period |
3,990,518 | 4,603 | 309 | 818 | | 5,730 | ||||||||||||||||||||
| Balance at February 28, 2017 (unaudited) |
14,702,556 | 66,576 | 309 | 5,607 | (50,106) | 22,386 | ||||||||||||||||||||
| Balance at March 31, 2017 |
14,702,556 | 66,576 | 309 | 5,693 | (50,875) | 21,703 | ||||||||||||||||||||
See accompanying notes to financial statements.
F-24
Table of Contents
ACASTI PHARMA INC.
Statements of Changes in Equity, Continued
Thirteen-month and one-month periods ended March 31, 2017, twelve-month period ended February 28, 2017 and years ended February 29, 2016 and February 28, 2015
| Share capital |
Other | Contributed | ||||||||||||||||||||||||
| Notes | Amount | Dollar | equity | surplus | Deficit | Total | ||||||||||||||||||||
| (thousands of Canadian dollars) | $ | $ | $ | $ | $ | |||||||||||||||||||||
| Balance, February 28, 2015 |
10,644,440 | 61,628 | | 4,911 | (33,311) | 33,228 | ||||||||||||||||||||
| Net loss and total comprehensive loss for the year |
| | | | (6,317) | (6,317) | ||||||||||||||||||||
| 10,644,440 | 61,628 | | 4,911 | (39,628) | 26,911 | |||||||||||||||||||||
| Transactions with owners, recorded directly in equity |
||||||||||||||||||||||||||
| Contributions by and distributions to equity holders |
||||||||||||||||||||||||||
| Share-based payment transactions |
15 | | | | 309 | | 309 | |||||||||||||||||||
| Issuance of shares |
12(c) | 50,000 | 101 | | (102) | | (1) | |||||||||||||||||||
| Share options exercised |
15 | 250 | 1 | | | | 1 | |||||||||||||||||||
| RSUs released |
17,348 | 243 | | (243) | | | ||||||||||||||||||||
| Total contributions by and distributions to equity holders |
67,598 | 345 | | (36) | | 309 | ||||||||||||||||||||
| Balance at February 29, 2016 |
10,712,038 | 61,973 | | 4,875 | (39,628) | 27,220 | ||||||||||||||||||||
| Share capital | Other | Contributed | ||||||||||||||||||||||||
| Notes | Amount | Dollar | equity | surplus | Deficit | Total | ||||||||||||||||||||
| (thousands of Canadian dollars) | $ | $ | $ | $ | $ | |||||||||||||||||||||
| Balance, February 28, 2014 |
61,027 | 407 | 3,502 | (31,656) | 33,280 | |||||||||||||||||||||
| 10,586,258 | ||||||||||||||||||||||||||
| Net loss and total comprehensive loss for the year |
| | | | (1,655) | (1,655) | ||||||||||||||||||||
| 61,027 | 407 | 3,502 | (33,311) | 31,625 | ||||||||||||||||||||||
| 10,586,258 | ||||||||||||||||||||||||||
| Transactions with owners, recorded directly in equity |
||||||||||||||||||||||||||
| Contributions by and distributions to equity holders |
||||||||||||||||||||||||||
| Share-based payment transactions |
15 | | | | 1,553 | | 1,553 | |||||||||||||||||||
| Share options exercised |
15 | 20,000 | 50 | | | | 50 | |||||||||||||||||||
| RSUs released |
38,182 | 551 | | (551) | | | ||||||||||||||||||||
| Expiration of warrants |
| | (407) | 407 | | | ||||||||||||||||||||
| Total contributions by and distributions to equity holders |
58,182 | 601 | (407) | 1,409 | | 1,603 | ||||||||||||||||||||
| Balance at February 28, 2015 |
10,644,440 | 61,628 | | 4,911 | (33,311) | 33,228 | ||||||||||||||||||||
See accompanying notes to financial statements.
F-25
Table of Contents
Thirteen-month and one-month periods ended March 31, 2017, twelve-month period ended February 28, 2017 and years ended February 29, 2016 and February 28, 2015
|
Thirteen-months ended |
Month ended | Twelve-months ended |
Year ended | Year ended | ||||||||||||||||||||
| Notes | March 31, 2017 |
March 31, 2017 |
February 28, 2017 |
February 29, 2016 |
February 28, 2015 |
|||||||||||||||||||
| (Unaudited) | (Unaudited) | |||||||||||||||||||||||
| (thousands of Canadian dollars) | $ | $ | $ | $ | $ | |||||||||||||||||||
| Cash flows used in operating activities: |
||||||||||||||||||||||||
| Net loss for the period |
(11,247) | (769) | (10,478) | (6,317) | (1,655) | |||||||||||||||||||
| Adjustments: |
||||||||||||||||||||||||
| Depreciation of equipment |
7 | 221 | 32 | 189 | 59 | 4 | ||||||||||||||||||
| Amortization of intangible assets |
8 | 2,517 | 194 | 2,323 | 2,336 | 2,331 | ||||||||||||||||||
| Impairment loss related to intangible assets |
8 | | | | 339 | | ||||||||||||||||||
| Stock-based compensation |
15 | 674 | 86 | 588 | 309 | 1,553 | ||||||||||||||||||
| Net financial expenses (income) |
14 | 166 | 51 | 115 | (3,295) | (10,740) | ||||||||||||||||||
| Realized foreign exchange gain (loss) |
48 | (12) | 60 | 36 | 3 | |||||||||||||||||||
| Deferred income tax recovery |
(129) | | (129) | | | |||||||||||||||||||
| (7,750) | (418) | (7,332) | (6,533) | (8,504) | ||||||||||||||||||||
| Changes in non-cash operating items |
17 | 792 | (328) | 1,120 | (41) | 1,306 | ||||||||||||||||||
| Net cash used in operating activities |
(6,958) | (746) | (6,212) | (6,574) | (7,198) | |||||||||||||||||||
| Cash flows from (used in) investing activities: |
||||||||||||||||||||||||
| Interest received |
150 | 4 | 146 | 114 | 41 | |||||||||||||||||||
| Acquisition of equipment |
7, 17 | (2,527) | (24) | (2,503) | (276) | (35) | ||||||||||||||||||
| Acquisition of intangible assets |
8 | | | | (92) | (51) | ||||||||||||||||||
| Acquisition of short-term investments |
(12,765) | | (12,765) | (11,954) | (14,478) | |||||||||||||||||||
| Maturity of short-term investments |
22,030 | | 22,030 | 20,437 | 22,150 | |||||||||||||||||||
| Net cash (used in) investing activities |
6,888 | (20) | 6,908 | 8,229 | 7,627 | |||||||||||||||||||
| Cash flows from (used in) financing activities: |
||||||||||||||||||||||||
| Net proceeds from public offering |
12(b) | 5,010 | (34) | 5,044 | | | ||||||||||||||||||
| Net proceeds from private placement |
11, 12(c) | 1,872 | (10) | 1,882 | | | ||||||||||||||||||
| Proceeds from exercise of warrants and options |
| | | | 50 | |||||||||||||||||||
| Share issue costs |
12(d) | | | | (1) | | ||||||||||||||||||
| Interest paid |
(18) | | (18) | (2) | (4) | |||||||||||||||||||
| Net cash from (used in) financing activities |
|
6,864 | (44) | 6,908 | (3) | 46 | ||||||||||||||||||
| Foreign exchange (loss) gain on cash and cash equivalents held in foreign currencies |
(49) | 9 | (58) | 64 | 160 | |||||||||||||||||||
| Net increase (decrease) in cash and cash equivalents |
6,745 | (801) | 7,546 | 1,716 | 635 | |||||||||||||||||||
| Cash and cash equivalents, beginning of period |
3,027 | 10,573 | 3,027 | 1,311 | 676 | |||||||||||||||||||
| Cash and cash equivalents, end of period |
9,772 | 9,772 | 10,573 | 3,027 | 1,311 | |||||||||||||||||||
| Cash and cash equivalents is comprised of: |
||||||||||||||||||||||||
| Cash |
6,778 | 6,778 | 7,584 | 3,027 | 1,311 | |||||||||||||||||||
| Cash equivalents |
2,994 | 2,994 | 2,989 | | | |||||||||||||||||||
See accompanying notes to financial statements.
F-26
Table of Contents
Thirteen-month and one-month periods ended March 31, 2017, twelve-month period ended February 28, 2017 and years ended February 29, 2016 and February 28, 2015
(thousands of Canadian dollars, except where noted and for share and per share amounts)
| 1. | Reporting entity |
Acasti Pharma Inc. (Acasti or the Corporation) is incorporated under the Business Corporations Act (Québec) (formerly Part 1A of the Companies Act (Québec)). The Corporation is domiciled in Canada and its registered office is located at 545, Promenade du Centropolis, Laval, Québec, H7T 0A3. Neptune Technologies and Bioressources Inc. (Neptune or the parent) currently owns approximately 34% of the issued and outstanding Class A shares (Common Shares) of the Corporation. The Corporation, Neptune and Biodroga Nutraceuticals Inc., a subsidiary of Neptune, are collectively referred to as the Group.
Pursuant to a license agreement entered into with Neptune in August 2008, as amended, Acasti has been granted an exclusive worldwide license to use Neptunes intellectual property to develop, clinically study and market new pharmaceutical products to treat human cardiovascular conditions. Neptunes intellectual property is related to the extraction of ingredients from marine biomasses, such as krill. The eventual products are aimed at applications in the prescription drug, over-the-counter medicine and medical foods markets. In December 2012, the Corporation entered into a prepayment agreement with Neptune pursuant to which the Corporation exercised its option under the License Agreement to pay in advance all of the future royalties payable under the license which was exercised in fiscal 2014. As a result of the royalty prepayment, Acasti is no longer required to pay any royalties to Neptune under the License Agreement during its term for the use of the intellectual property under license. The license allows Acasti to exploit the intellectual property rights in order to develop novel active pharmaceutical ingredients (APIs) into commercial products for the prescription drugs and the medical food markets.
The Corporation is subject to a number of risks associated with the conduct of its clinical program and its results, the establishment of strategic alliances and the successful development of new pharmaceutical products and their marketing. The Corporation has incurred significant operating losses and negative cash flows from operations since inception. To date, the Corporation has financed its operations through the public offering and private placement of Common Shares and convertible debt, the proceeds from research grants and research tax credits, and the exercises of warrants, rights and options. To achieve the objectives of its business plan, Acasti plans to raise the necessary funds through additional securities offerings and the establishment of strategic alliances as well as additional research grants and research tax credits. The Corporation anticipates that the products developed by the Corporation will require approval from the U.S Food and Drug Administration and equivalent regulatory organizations in other countries before their sale can be authorized. The ability of the Corporation to ultimately achieve profitable operations is dependent on a number of factors outside of the Corporations control.
| 2. | Basis of preparation |
| (a) | Statement of compliance: |
These financial statements have been prepared in accordance with International Financial Reporting Standards (IFRS) as issued by the International Accounting Standards Board (IASB). Beginning in fiscal 2017, the Corporations fiscal year end is on March 31. Fiscal 2017 is a transition year, and includes thirteen months of operations, beginning on March 1, 2016 and ending on March 31, 2017. As a result, the above financial statements and corresponding notes to financial statements include two unaudited periods: the one-month period ended March 31, 2017 and the twelve-month period ended February 28, 2017. The Canadian Securities regulator permits, in the transition year, the presentation of a thirteen-month period for the financial year ended March 31, 2017.
The financial statements were approved by the Board of Directors on June 6, 2017.
| (b) | Basis of measurement: |
The financial statements have been prepared on the historical cost basis, except for:
| | Stock-based compensation which is measured pursuant to IFRS 2, Share-based payments (Note 3(e) (ii)); and, |
| | Derivative warrant liabilities measured at fair value on a recurring basis (Note 10). |
F-27
Table of Contents
ACASTI PHARMA INC.
Notes to Financial Statements
Thirteen-month and one-month periods ended March 31, 2017, twelve-month period ended February 28, 2017 and years ended February 29, 2016 and February 28, 2015
(thousands of Canadian dollars, except where noted and for share and per share amounts)
| 2. | Basis of preparation (continued): |
| (c) | Going concern uncertainty: |
The Corporation has incurred operating losses and negative cash flows from operations since inception. The Corporations current assets of $10.2 million as at March 31, 2017 include cash and cash equivalents totalling $9.8 million, mainly generated by the net proceeds from the Public Offering and Private Placement completed on February 21, 2017 as well as the public offering completed on December 3, 2013 and private offering completed on February 7, 2014 (the Previous Offerings). The Corporations liabilities total $3.8 million at March 31, 2017 and are comprised primarily of $2.1 million in amounts due to or accrued for creditors, $1.4 million for unsecured convertible debentures and $0.2 million for derivative warrant liabilities. The Corporations current assets as at this date are projected to be significantly less than needed to support the current liabilities as at that date when combined with the projected level of expenses for the next twelve months, including not only the preparation for, but the planned initiation of the Phase 3 clinical study program for its drug candidate, CaPre. Additional funds will also be needed for the expected expenses for the total CaPre Phase 3 research and development phase beyond the next twelve months. In addition to having raised additional funds during the thirteen-month period ended March 31, 2017, the Corporation is working towards development of strategic partner relationships and plans to raise additional funds in the future, but there can be no assurance as to when or whether Acasti will complete any financing or strategic collaborations. In particular, raising financing is subject to market conditions and is not within the Corporations control. Additionally, although the Corporation intends to continue to rely on the support of Neptune for a portion of its general and administrative needs, the continuance of this support is outside of the Corporations control. If the Corporation does not raise additional funds, find one or more strategic partners or does not receive the continued support from its parent, it may not be able to realize its assets and discharge its liabilities in the normal course of business. As a result, there exists a material uncertainty that casts substantial doubt about the Corporations ability to continue as a going concern and, therefore, realize its assets and discharge its liabilities in the normal course of business. The Corporation currently has no other arranged sources of financing.
The financial statements have been prepared on a going concern basis, which assumes the Corporation will continue its operations in the foreseeable future and will be able to realize its assets and discharge its liabilities and commitments in the ordinary course of business. These financial statements do not include any adjustments to the carrying values and classification of assets and liabilities and reported expenses that may be necessary if the going concern basis was not appropriate for these financial statements. If the Corporation was unable to continue as a going concern, material write-downs to the carrying values of the Corporations assets, including the intangible asset, could be required.
| (d) | Functional and presentation currency: |
These financial statements are presented in Canadian dollars, which is the Corporations functional currency.
| (e) | Use of estimates and judgments: |
The preparation of the financial statements in conformity with IFRS requires management to make judgments, estimates and assumptions that affect the application of accounting policies and the reported amounts of assets, liabilities, income and expenses. Actual results may differ from these estimates.
Estimates are based on managements best knowledge of current events and actions that the Corporation may undertake in the future. Estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimates are revised and in any future periods affected.
Critical judgments in applying accounting policies that have the most significant effect on the amounts recognized in the financial statements include the following:
| | Identification of triggering events indicating that the intangible assets might be impaired. |
| | The use of the going concern basis of preparation of the financial statements. At the end of each reporting period, management assesses the basis of preparation of the financial statements (Note 2(c)). |
F-28
Table of Contents
ACASTI PHARMA INC.
Notes to Financial Statements
Thirteen-month and one-month periods ended March 31, 2017, twelve-month period ended February 28, 2017 and years ended February 29, 2016 and February 28, 2015
(thousands of Canadian dollars, except where noted and for share and per share amounts)
| 2. | Basis of preparation (continued): |
| (e) | Use of estimates and judgments (continued): |
Assumptions and estimation uncertainties that have a significant risk of resulting in a material adjustment within the next financial year include the following:
| ● | Determination of the recoverable amount of the Corporations cash generating unit (CGU). |
| ● | Measurement of derivative warrant liabilities (note 10) and stock-based compensation (note 15). |
Also, management uses judgment to determine which research and development (R&D) expenses qualify for R&D tax credits and in what amounts. The Corporation recognizes the tax credits once it has reasonable assurance that they will be realized. Recorded tax credits are subject to review and approval by tax authorities and therefore, could be different from the amounts recorded.
| 3. | Significant accounting policies: |
The accounting policies set out below have been applied consistently to all periods presented in these financial statements.
| (a) | Financial instruments: |
A financial instrument is any contract that gives rise to a financial asset of one party and a financial liability or equity instrument of another party.
| (i) | Non-derivative financial assets: |
The Corporation has the following non-derivative financial assets: cash, cash equivalents, short-term investments and receivables. The Corporation determines the classification of its financial assets at initial recognition. The subsequent measurement of financial assets depends on their classification.
Financial assets and liabilities are offset and the net amount presented in the statements of financial position when, and only when, the Corporation has a legal right to offset the amounts and intends either to settle on a net basis or to realize the asset and settle the liability simultaneously.
Loans and receivables
The classification loans and receivables comprises financial assets with fixed or determinable payments that are not quoted in an active market. Such assets are recognized initially at fair value plus any directly attributable transaction costs. Subsequent to initial recognition, loans and receivables are measured at amortized cost using the effective interest method, less any impairment losses.
Cash, cash equivalents, short-term investments and receivables with maturities of less than one year are classified as loans and receivables.
Cash and cash equivalents comprise cash balances and highly liquid investments purchased three months or less from maturity.
| (ii) | Non-derivative financial liabilities: |
The Corporation has the following non-derivative financial liabilities: trade and other payables, payable to parent corporation and unsecured convertible debentures. Such financial liabilities are recognized initially at fair value plus any directly attributable transaction costs. Subsequent to initial recognition, these financial liabilities are measured at amortized cost using the effective interest method.
The Corporation derecognizes a financial liability when its contractual obligations are discharged, cancelled or expire.
F-29
Table of Contents
ACASTI PHARMA INC.
Notes to Financial Statements
Thirteen-month and one-month periods ended March 31, 2017, twelve-month period ended February 28, 2017 and years ended February 29, 2016 and February 28, 2015
(thousands of Canadian dollars, except where noted and for share and per share amounts)
| 3. | Significant accounting policies (continued): |
| (a) | Financial instruments (continued): |
| (iii) | Compound financial instruments: |
Compound financial instruments are instruments that can be converted to share capital at the option of the holder, and the number of shares to be issued is fixed.
The unsecured convertible debentures are compound instruments and have been separated into liability and equity components. The liability component is recognized initially at the fair value of a similar liability that does not have an equity conversion option. The equity component is recognized initially as the difference between the fair value of the compound financial instrument as a whole and the fair value of the liability component. Any directly attributable transaction costs are allocated to the liability and equity components in proportion to their initial carrying amounts. Subsequent to initial recognition, the liability component of a compound financial instrument is measured at amortized cost using the effective interest method. The equity component of a compound financial instrument is not remeasured subsequent to initial recognition.
| (iv) | Share capital: |
Common Shares
Class A Common Shares are classified as equity. Incremental costs directly attributable to the issue of Common Shares and share options are recognized as a deduction from share capital, net of any tax effects.
| (v) | Derivative financial instruments: |
The Corporation has issued liability-classified derivatives over its own equity. Derivatives are recognized initially at fair value; attributable transaction costs are recognized in profit and loss as incurred. Subsequent to initial recognition, derivatives are measured at fair value, and all changes in their fair value are recognized immediately in profit or loss.
| (vi) | Other equity instruments: |
Warrants, options and rights over the Corporations equity issued outside of share-based payment transactions that do not meet the definition of a liability instrument are recognized in equity.
| (b) | Equipment: |
| (i) | Recognition and measurement: |
Equipment is measured at cost less accumulated depreciation and accumulated impairment losses, if any.
Cost includes expenditures that are directly attributable to the acquisition of the asset, including all costs incurred in bringing the asset to its present location and condition.
Purchased software that is integral to the functionality of the related equipment is capitalized as part of that equipment.
Gains and losses on disposal of equipment are determined by comparing the proceeds from disposal with the carrying amount of equipment, and are recognized net within other income or expenses in profit or loss.
| (ii) | Subsequent costs: |
The cost of replacing a part of an equipment is recognized in the carrying amount of the item if it is probable that the future economic benefits embodied within the part will flow to the Corporation, and its cost can be measured reliably. The carrying amount of the replaced part is derecognized. The costs of the day-to-day servicing of equipment are recognized in profit or loss as incurred.
F-30
Table of Contents
ACASTI PHARMA INC.
Notes to Financial Statements
Thirteen-month and one-month periods ended March 31, 2017, twelve-month period ended February 28, 2017 and years ended February 29, 2016 and February 28, 2015
(thousands of Canadian dollars, except where noted and for share and per share amounts)
| 3. | Significant accounting policies (continued): |
| (b) | Equipment (continued): |
| (iii) | Depreciation: |
Depreciation is recognized in profit or loss on either a straight-line basis or a declining basis over the estimated useful lives of each part of an item of equipment, since this most closely reflects the expected pattern of consumption of the future economic benefits embodied in the asset. Items of equipment are depreciated from the date that they are available for use or, in respect of assets not yet in service, from the date they are ready for their intended use.
The estimated useful lives and rates for the current and comparative periods are as follows:
| Assets | Method | Period/Rate | ||
| Furniture and office equipment |
Declining balance | 20% to 30% | ||
| Computer equipment |
Declining balance | 30% | ||
| Laboratory equipment |
Declining balance | 30% | ||
| Production equipment |
Straight-line | 10 years | ||
Depreciation methods, useful lives and residual values are reviewed at each financial year-end and adjusted prospectively if appropriate.
| (c) | Intangible assets: |
| (i) | Research and development: |
Expenditure on research activities, undertaken with the prospect of gaining new scientific or technical knowledge and understanding, is recognized in profit or loss as incurred.
Development activities involve a plan or design for the production of new or substantially improved products and processes. Development expenditure is capitalized only if development costs can be measured reliably, the product or process is technically and commercially feasible, future economic benefits are probable, and the Corporation intends to and has sufficient resources to complete development and to use or sell the asset. The expenditure capitalized includes the cost of materials, direct labour, overhead costs that are directly attributable to preparing the asset for its intended use, and borrowing costs on qualifying assets. Other development expenditures are recognized in profit or loss as incurred.
Capitalized development expenditure is measured at cost less accumulated amortization and accumulated impairment losses. As of the reporting periods presented, the Corporation has not capitalized any development expenditure.
| (ii) | Other intangible assets: |
Patent costs
Patents for technologies that are no longer in the research phase are recorded at cost. Patent costs include legal fees to obtain patents and patent application fees. When the technology is still in the research and development phase, those costs are expensed as incurred.
Licenses
Licenses that are acquired by the Corporation and have finite useful lives are measured at cost less accumulated amortization and accumulated impairment losses.
F-31
Table of Contents
ACASTI PHARMA INC.
Notes to Financial Statements
Thirteen-month and one-month periods ended March 31, 2017, twelve-month period ended February 28, 2017 and years ended February 29, 2016 and February 28, 2015
(thousands of Canadian dollars, except where noted and for share and per share amounts)
| 3. | Significant accounting policies (continued): |
| (c) | Intangible assets (continued): |
| (iii) | Subsequent expenditure: |
Subsequent expenditure is capitalized only when it increases the future economic benefits embodied in the specific asset to which it relates. All other expenditures, including expenditure on internally generated goodwill and brands, are recognized in profit or loss as incurred.
| (iv) | Amortization: |
Amortization is calculated over the cost of the asset less its residual value.
Amortization is recognized in profit or loss on a straight-line basis over the estimated useful lives of intangible assets from the date that they are available for use, since this most closely reflects the expected pattern of consumption of the future economic benefits embodied in the asset. The estimated useful lives for the current and comparative periods are as follows:
| Assets | Period | |
| Patents |
20 years | |
| License |
8 to 14 years | |
| (d) | Impairment: |
| (i) | Financial assets: |
A financial asset not carried at fair value through profit or loss is assessed at each reporting date to determine whether there is objective evidence that it is impaired. A financial asset is impaired if objective evidence, such as default or delinquency by a debtor, indicates that a loss event has occurred after the initial recognition of the asset, and that the loss event had a negative effect on the estimated future cash flows of that asset that can be estimated reliably.
An impairment loss in respect of a financial asset measured at amortized cost is calculated as the difference between its carrying amount and the present value of the estimated future cash flows discounted at the assets original effective interest rate. Losses are recognized in profit or loss and reflected in an allowance account against the financial asset. When a subsequent event causes the amount of impairment loss to decrease, the decrease in impairment loss is reversed through profit or loss.
| (ii) | Non-financial assets: |
The carrying amounts of the Corporations non-financial assets are reviewed at each reporting date to determine whether there is any indication of impairment. If any such indication exists, then the assets recoverable amount is estimated.
The recoverable amount of an asset or cash-generating unit is the greater of its value in use and its fair value less costs to sell. In assessing value in use, the estimated future cash flows are discounted to their present value using a pre-tax discount rate that reflects current market assessments of the time value of money and the risks specific to the asset. For the purpose of impairment testing, assets that cannot be tested individually are grouped together into the smallest group of assets that generates cash inflows from continuing use that are largely independent of the cash inflows of other assets or groups of assets (the cash-generating unit, or CGU).
F-32
Table of Contents
ACASTI PHARMA INC.
Notes to Financial Statements
Thirteen-month and one-month periods ended March 31, 2017, twelve-month period ended February 28, 2017 and years ended February 29, 2016 and February 28, 2015
(thousands of Canadian dollars, except where noted and for share and per share amounts)
| 3. | Significant accounting policies (continued): |
| (d) | Impairment (continued): |
| (ii) | Non-financial assets (continued): |
The Corporations corporate assets do not generate separate cash inflows. If there is an indication that a corporate asset may be impaired, then the recoverable amount is determined for the CGU to which the corporate asset belongs.
An impairment loss is recognized if the carrying amount of an asset or its CGU exceeds its estimated recoverable amount. Impairment losses are recognized in profit or loss.
Impairment losses recognized in prior years are assessed at each reporting date for any indications that the loss has decreased or no longer exists. An impairment loss is reversed if there has been a change in the estimates used to determine the recoverable amount. An impairment loss is reversed only to the extent that the assets carrying amount does not exceed the carrying amount that would have been determined, net of depreciation or amortization, if no impairment loss had been recognized.
| (e) | Employee benefits: |
| (i) | Short-term employee benefits: |
Short-term employee benefit obligations are measured on an undiscounted basis and are expensed as the related service is provided.
A liability is recognized for the amount expected to be paid under short-term cash bonus or profit-sharing plans if the Corporation has a present legal or constructive obligation to pay this amount as a result of past service provided by the employee, and the obligation can be estimated reliably.
| (ii) | Share-based payment transactions: |
The grant date fair value of share-based payment awards granted to employees is recognized as an employee expense, with a corresponding increase in contributed surplus, over the period that the employees unconditionally become entitled to the awards. The grant date fair value takes into consideration market performance conditions when applicable. The amount recognized as an expense is adjusted to reflect the number of awards for which the related service and non-market vesting conditions are expected to be met, such that the amount ultimately recognized as an expense is based on the number of awards that do meet the related service and non-market performance conditions at the vesting date.
Share-based payment arrangements in which the Corporation receives goods or services as consideration for its own equity instruments are accounted for as equity-settled share-based payment transactions, regardless of how the equity instruments are obtained by the Corporation.
| (iii) | Termination benefits: |
Termination benefits are recognized as an expense when the Corporation is committed demonstrably, without realistic possibility of withdrawal, to a formal detailed plan to either terminate employment before the normal retirement date, or to provide termination benefits as a result of an offer made to encourage voluntary redundancy. Termination benefits for voluntary redundancies are recognized as an expense if the Corporation has made an offer of voluntary redundancy, it is probable that the offer will be accepted, and the number of acceptances can be estimated reliably. If benefits are payable more than 12 months after the reporting year, then they are discounted to their present value.
F-33
Table of Contents
ACASTI PHARMA INC.
Notes to Financial Statements
Thirteen-month and one-month periods ended March 31, 2017, twelve-month period ended February 28, 2017 and years ended February 29, 2016 and February 28, 2015
(thousands of Canadian dollars, except where noted and for share and per share amounts)
| 3. | Significant accounting policies (continued): |
| (f) | Provisions: |
A provision is recognized if, as a result of a past event, the Corporation has a present legal or constructive obligation that can be estimated reliably, and it is probable that an outflow of economic benefits will be required to settle the obligation. Provisions are determined by discounting the expected future cash flows at a pre-tax rate that reflects current market assessments of the time value of money and the risks specific to the liability. The unwinding of the discount is recognized as finance cost.
| (i) | Onerous contracts: |
A provision for onerous contracts is recognized when the expected benefits to be derived by the Corporation from a contract are lower than the unavoidable cost of meeting its obligations under the contract. The provision is measured at the present value of the lower of the expected cost of terminating the contract and the expected net cost of continuing with the contract. Before a provision is established, the Corporation recognizes any impairment loss on the assets associated with that contract.
| (ii) | Contingent liability: |
A contingent liability is a possible obligation that arises from past events and of which the existence will be confirmed only by the occurrence or non-occurrence of one or more uncertain future events not within the control of the Corporation; or a present obligation that arises from past events (and therefore exists), but is not recognized because it is not probable that a transfer or use of assets, provision of services or any other transfer of economic benefits will be required to settle the obligation; or the amount of the obligation cannot be estimated reliably.
| (g) | Government grants: |
Government grants are recorded as a reduction of the related expense or cost of the asset acquired. Government grants are recognized when there is reasonable assurance that the Corporation has met the requirements of the approved grant program and there is reasonable assurance that the grant will be received.
Grants that compensate the Corporation for expenses incurred are recognized in profit or loss in reduction thereof on a systematic basis in the same years in which the expenses are recognized. Grants that compensate the Corporation for the cost of an asset are recognized in profit or loss on a systematic basis over the useful life of the asset.
| (h) | Lease payments: |
Payments made under operating leases are recognized in profit or loss on a straight-line basis over the term of the lease. Lease incentives received are recognized as an integral part of the total lease expense, over the term of the lease.
| (i) | Foreign currency: |
Transactions in foreign currencies are translated into the functional currency at exchange rates at the dates of the transactions. Monetary assets and liabilities denominated in foreign currencies at the reporting date are retranslated to the functional currency at the exchange rate at that date. The foreign currency gain or loss on monetary items is the difference between amortized cost in the functional currency at the beginning of the period, adjusted for effective interest and payments during the period, and the amortized cost in foreign currency translated at the exchange rate at the end of the reporting period. Foreign currency differences arising on retranslation are recognized in profit or loss.
F-34
Table of Contents
ACASTI PHARMA INC.
Notes to Financial Statements
Thirteen-month and one-month periods ended March 31, 2017, twelve-month period ended February 28, 2017 and years ended February 29, 2016 and February 28, 2015
(thousands of Canadian dollars, except where noted and for share and per share amounts)
| 3. | Significant accounting policies (continued): |
| (j) | Finance income and finance costs: |
Finance income comprises interest income on funds invested. Interest income is recognized as it accrues in profit or loss, using the effective interest method.
Finance costs comprise interest expense and accretion on borrowings, unwinding of the discount on provisions and impairment losses recognized on financial assets. Borrowing costs that are not directly attributable to the acquisition, construction or production of a qualifying asset are recognized in profit or loss using the effective interest method.
Foreign currency gains and losses are reported on a net basis.
The Corporation recognizes interest income as a component of investing activities and interest expense as a component of financing activities in the statements of cash flows.
| (k) | Income tax: |
Income tax expense comprises current and deferred taxes. Current and deferred taxes are recognized in profit or loss except to the extent that they relate to items recognized directly in equity or in other comprehensive income.
Current tax is the expected tax payable or receivable on the taxable income or loss for the year, using tax rates enacted or substantively enacted at the reporting date, and any adjustment to tax payable in respect of previous years.
Deferred tax is recognized in respect of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for taxation purposes. Deferred tax is not recognized for temporary differences arising from the initial recognition of assets or liabilities in a transaction that is not a business combination and that affects neither accounting nor taxable profit or loss. Deferred tax is measured at the tax rates that are expected to be applied to temporary differences when they reverse, based on the laws that have been enacted or substantively enacted by the reporting date. Deferred tax assets and liabilities are offset if there is a legally enforceable right to offset current tax liabilities and assets, and they relate to income taxes levied by the same tax authority on the same taxable entity, or on different tax entities, but they intend to settle current tax liabilities and assets on a net basis or their tax assets and liabilities will be realized simultaneously. A deferred tax asset is recognized for unused tax losses, tax credits and deductible temporary differences, to the extent that it is probable that future taxable profits will be available against which they can be utilized. Deferred tax assets are reviewed at each reporting date and are reduced to the extent that it is no longer probable that the related tax benefit will be realized.
| (l) | Earnings per share: |
The Corporation presents basic and diluted earnings per share (EPS) data for its Class A shares (or Common Shares). Basic EPS is calculated by dividing the profit or loss attributable to the holders of Class A shares (Common Shares) of the Corporation by the weighted average number of Common Shares outstanding during the year, adjusted for own shares held. Diluted EPS is determined by adjusting the profit or loss attributable to the holders of Class A shares (Common Shares) and the weighted average number of Class A shares (Common Shares) outstanding adjusted for the effects of all dilutive potential Common Shares, which comprise warrants, rights and share options granted to employees.
| (m) | Segment reporting: |
An operating segment is a component of the Corporation that engages in business activities from which it may earn revenues and incur expenses. The Corporation has one reportable operating segment: the development and commercialization of pharmaceutical applications of its licensed rights for cardiovascular diseases. The majority of the Corporations assets are located in Canada, while one major production unit, with a carrying value of $2,394, is located in France.
F-35
Table of Contents
ACASTI PHARMA INC.
Notes to Financial Statements
Thirteen-month and one-month periods ended March 31, 2017, twelve-month period ended February 28, 2017 and years ended February 29, 2016 and February 28, 2015
(thousands of Canadian dollars, except where noted and for share and per share amounts)
| 3. | Significant accounting policies (continued): |
| (n) | Change in accounting policy: |
Future accounting change:
The following new standards, and amendments to standards and interpretations, are not yet effective for the period ended March 31, 2017, and have not been applied in preparing these financial statements.
New standards and interpretations not yet adopted:
| (i) | Financial instruments: |
On July 24, 2014, the International Accounting Standards Board (IASB) issued the final version of IFRS 9, Financial Instruments, which addresses the classification and measurement of financial assets and liabilities, impairment and hedge accounting, replacing IAS 39, Financial Instruments: Recognition and Measurement. IFRS 9 is effective for annual periods beginning on or after January 1, 2018, with earlier adoption permitted. The Corporation intends to adopt IFRS 9 in its financial statements for the annual period beginning on April 1, 2018. The Corporation has not yet assessed the impact of adoption of IFRS 9, and does not intend to early adopt IFRS 9 in its financial statements.
| (ii) | Amendments to IFRS 2 Classification and Measurement of Share-Based Payment Transactions: |
On June 20, 2016, the IASB issued amendments to IFRS 2, Share-Based Payment, clarifying how to account for certain types of share-based payment transactions. The amendments apply for annual periods beginning on or after January 1, 2018. Earlier application is permitted. As a practical simplification, the amendments can be applied prospectively. Retrospective, or early application is permitted if information is available without the use of hindsight. The amendments provide requirements on the accounting for: the effects of vesting and non-vesting conditions on the measurement of cash-settled share-based payments; share-based payment transactions with a net settlement feature for withholding tax obligations; and a modification to the terms and conditions of a share-based payment that changes the classification of the transaction from cash-settled to equity-settled. The Corporation intends to adopt the amendments to IFRS 2 in its financial statements for the annual period beginning on April 1, 2018. The Corporation has not yet assessed the impact of adoption of the amendments of IFRS 2, and does not intend to early adopt these amendments in its financial statements.
| 4. | Receivables: |
| Notes | March 31, 2017 | February 28, 2017 (Unaudited) |
February 29, 2016 | |||||||||||||
| $ | $ | $ | ||||||||||||||
| Sales tax receivables |
89 | 83 | 182 | |||||||||||||
| Government assistance and tax credits receivable |
6 | 115 | 81 | 217 | ||||||||||||
| Other receivables |
2 | 2 | | |||||||||||||
| 206 | 166 | 399 | ||||||||||||||
F-36
Table of Contents
ACASTI PHARMA INC.
Notes to Financial Statements
Thirteen-month and one-month periods ended March 31, 2017, twelve-month period ended February 28, 2017 and years ended February 29, 2016 and February 28, 2015
(thousands of Canadian dollars, except where noted and for share and per share amounts)
| 5. | Related parties: |
| (a) | Administrative and research and development expenses: |
The Corporation was charged by Neptune for the purchase of research supplies and for certain costs incurred by Neptune for the benefit of the Corporation, as follows:
|
Thirteen-months ended |
Month ended |
Twelve-months ended |
Year ended | Year ended | ||||||
| March 31, 2017 |
March 31, 2017 |
February 28, 2017 (Unaudited) |
February 29, 2016 |
February 28, 2015 |
||||||
| $ | $ | $ | $ | $ | ||||||
| Research and development expenses |
60 | 1 | 59 | 371 | 344 | |||||
| General and administrative expenses |
618 | 41 | 577 | 790 | 876 | |||||
| 678 | 42 | 636 | 1,161 | 1,220 | ||||||
The Corporation purchased from the parent company research and development supplies totaling $113, of which $73 as at March 31, 2017 and as at February 28, 2017 (unaudited) is recorded in prepaid expenses and will be expensed as used.
Where Neptune incurs specific incremental costs for the benefit of the Corporation, it charges those amounts directly. Costs that benefit more than one entity of the Group are charged by allocating a fraction of costs incurred by Neptune that is commensurate to the estimated fraction of services or benefits received by each entity for those items.
These charges do not represent all charges incurred by Neptune that may have benefited the Corporation. Also, these charges do not necessarily represent the cost that the Corporation would otherwise need to incur, should it not receive these services or benefits through the shared resources of Neptune.
| (b) | Interest revenue: |
On January 7, 2016 Neptune announced the acquisition of Biodroga Nutraceuticals Inc. As part of this transaction, the Corporation pledged an amount of $2 million (Committed Funds) to partly guarantee the financing for the said transaction (Pledge Agreement). Neptune had agreed to pay Acasti an annual fee on the Committed Funds outstanding at an annual rate of 9% during the first six months and 11% for the remaining term of the Pledge Agreement. On September 20, 2016, Neptune fully released the pledged amount. The Corporation recognized interest revenue in the amount of $89 for the thirteen-month period ended March 31, 2017, nil (unaudited) for the month ended March 31, 2017, $89 (unaudited) for the twelve-month period ended February 28, 2017 and $27 for the year ended February 29, 2016.
| (c) | Payable to parent corporation: |
Payable to parent corporation, primarily for general and administrative shared services, has no specified maturity date for payment or reimbursement and does not bear interest.
| (d) | Key management personnel compensation: |
The key management personnel are the officers of the Corporation, the members of the Board of Directors of the Corporation and of the parent company. They control in the aggregate less than 2% of the voting shares of the Corporation (1% in 2016 and 2% in 2015).
F-37
Table of Contents
ACASTI PHARMA INC.
Notes to Financial Statements
Thirteen-month and one-month periods ended March 31, 2017, twelve-month period ended February 28, 2017 and years ended February 29, 2016 and February 28, 2015
(thousands of Canadian dollars, except where noted and for share and per share amounts)
| 5. | Related parties (continued): |
| (d) | Key management personnel compensation (continued): |
Key management personnel compensation includes the following for the thirteen-month and one-month periods ended March 31, 2017, twelve-month period ended February 28, 2017 and years ended February 29, 2016 and February 28, 2015:
|
Thirteen-months ended |
Month ended |
Twelve-months ended |
Year ended | Year ended | ||||||
| March 31, 2017 |
March 31, 2017 |
February 28, 2017 (Unaudited) |
February 29, 2016 |
February 28, 2015 |
||||||
| $ | $ | $ | $ | $ | ||||||
| Short-term benefits |
1,311 | 202 | 1,109 | 688 | 742 | |||||
| Severance |
| | | 103 | 175 | |||||
| Share-based compensation costs |
619 | 78 | 541 | 120 | 1,339 | |||||
| 1,930 | 280 | 1,650 | 911 | 2,256 | ||||||
| 6. | Government assistance: |
Government assistance is comprised of a government grant from the federal government and research and development investment tax credits receivable from the provincial government which relate to qualifiable research and development expenditures under the applicable tax laws. The amounts recorded as receivables are subject to a government tax audit and the final amounts received may differ from those recorded.
Unrecognized federal tax credits may be used to reduce future income tax and expire as follows:
| $ | ||
| 2029 |
11 | |
| 2030 |
30 | |
| 2031 |
45 | |
| 2032 |
431 | |
| 2033 |
441 | |
| 2034 |
436 | |
| 2035 |
519 | |
| 2036 |
286 | |
| 2037 |
251 | |
|
2,450 |
||
F-38
Table of Contents
ACASTI PHARMA INC.
Notes to Financial Statements
Thirteen-month and one-month periods ended March 31, 2017, twelve-month period ended February 28, 2017 and years ended February 29, 2016 and February 28, 2015
(thousands of Canadian dollars, except where noted and for share and per share amounts)
| 7. | Equipment: |
| Furniture and office equipment |
Computer equipment |
Laboratory equipment |
Production equipment |
Total | ||||||||||||||||
| $ | $ | $ | $ | $ | ||||||||||||||||
| Cost: |
||||||||||||||||||||
| Balance at February 28, 2014 |
59 | 3 | 25 | | 87 | |||||||||||||||
| Additions |
| | 35 | | 35 | |||||||||||||||
| Balance at February 28, 2015 |
59 | 3 | 60 | | 122 | |||||||||||||||
| Additions |
| | 276 | | 276 | |||||||||||||||
| Balance at February 29, 2016 |
59 | 3 | 336 | | 398 | |||||||||||||||
| Additions for the twelve-month period (Unaudited) |
| 8 | 186 | 2,578 | 2,772 | |||||||||||||||
| Balance at February 28, 2017 (Unaudited) |
59 | 11 | 522 | 2,578 | 3,170 | |||||||||||||||
| Additions for the one-month period (Unaudited) |
| | | 43 | 43 | |||||||||||||||
| Additions for the thirteen-month period |
| 8 | 186 | 2,621 | 2,815 | |||||||||||||||
| Balance at March 31, 2017 |
59 | 11 | 522 | 2,621 | 3,213 | |||||||||||||||
| Accumulated depreciation: |
||||||||||||||||||||
| Balance at February 28, 2014 |
45 | 3 | | | 48 | |||||||||||||||
| Depreciation for the year |
4 | | | | 4 | |||||||||||||||
| Balance at February 28, 2015 |
49 | 3 | | | 52 | |||||||||||||||
| Depreciation for the year |
3 | | 56 | | 59 | |||||||||||||||
| Balance at February 29, 2016 |
52 | 3 | 56 | | 111 | |||||||||||||||
| Depreciation for the twelve-month period (Unaudited) |
7 | 1 | 129 | 52 | 189 | |||||||||||||||
| Balance at February 28, 2017 (Unaudited) |
59 | 4 | 185 | 52 | 300 | |||||||||||||||
| Depreciation for the one-month period (Unaudited) |
| | 11 | 21 | 32 | |||||||||||||||
| Depreciation for thirteen-month period |
7 | 1 | 140 | 73 | 221 | |||||||||||||||
| Balance at March 31, 2017 |
59 | 4 | 196 | 73 | 332 | |||||||||||||||
| Net carrying amounts: |
||||||||||||||||||||
| February 29, 2016 |
7 | | 280 | | 287 | |||||||||||||||
| February 28, 2017 (Unaudited) |
| 7 | 337 | 2,526 | 2,870 | |||||||||||||||
| March 31, 2017 |
| 7 | 326 | 2,548 | 2,881 | |||||||||||||||
Depreciation expense for the thirteen-month and one-month periods ended March 31, 2017 and twelve-month period ended February 28, 2017 and years ended February 29, 2016 and February 28, 2015 has been recorded in research and development expenses in the statements of earnings and comprehensive loss.
F-39
Table of Contents
ACASTI PHARMA INC.
Notes to Financial Statements
Thirteen-month and one-month periods ended March 31, 2017, twelve-month period ended February 28, 2017 and years ended February 29, 2016 and February 28, 2015
(thousands of Canadian dollars, except where noted and for share and per share amounts)
| 8. | Intangible assets : |
| Patents | License | Total | ||||||||||
| $ | $ | $ | ||||||||||
| Cost: |
||||||||||||
| Balance at February 28, 2014 |
227 | 24,330 | 24,557 | |||||||||
| Additions |
51 | | 51 | |||||||||
| Balance at February 28, 2015 |
278 | 24,330 | 24,608 | |||||||||
| Additions |
84 | | 84 | |||||||||
| Balance at February 29,
2016, February 28, 2017 |
362 | 24,330 | 24,692 | |||||||||
| Accumulated amortization: |
||||||||||||
| Balance at February 28, 2014 |
1 | 4,780 | 4,781 | |||||||||
| Amortization for the year |
9 | 2,322 | 2,331 | |||||||||
| Balance at February 28, 2015 |
10 | 7,102 | 7,112 | |||||||||
| Amortization for the year |
13 | 2,323 | 2,336 | |||||||||
| Impairment loss |
339 | | 339 | |||||||||
| Balance at February 29, 2016 |
362 | 9,425 | 9,787 | |||||||||
| Amortization for the twelve-month period (Unaudited) |
| 2,323 | 2,323 | |||||||||
| Balance at February 28, 2017 (Unaudited) |
362 | 11,748 | 12,110 | |||||||||
| Amortization for the one-month period (Unaudited) |
| 194 | 194 | |||||||||
| Amortization for the thirteen-month period |
| 2,517 | 2,517 | |||||||||
| Balance at March 31, 2017 |
362 | 11,942 | 12,304 | |||||||||
| Net carrying amounts: |
||||||||||||
| February 29, 2016 |
| 14,905 | 14,905 | |||||||||
| February 28, 2017 (Unaudited) |
| 12,582 | 12,582 | |||||||||
| March 31, 2017 |
| 12,388 | 12,388 | |||||||||
Amortization expense and impairment loss for the thirteen-month and one-month periods ended March 31, 2017, the twelve-month period ended February 28, 2017 and years ended February 29, 2016 and February 28, 2015 have been recorded in research and development expenses in the statements of earnings and comprehensive loss.
F-40
Table of Contents
ACASTI PHARMA INC.
Notes to Financial Statements
Thirteen-month and one-month periods ended March 31, 2017, twelve-month period ended February 28, 2017 and years ended February 29, 2016 and February 28, 2015
(thousands of Canadian dollars, except where noted and for share and per share amounts)
| 9. | Trade and other payables: |
| March 31, 2017 |
February 28, 2017 (Unaudited) |
February 29, 2016 | ||||||||||
| $ | $ | $ | ||||||||||
| Trade payables |
259 | 534 | 375 | |||||||||
| Accrued liabilities and other payables |
1,354 | 1,372 | 543 | |||||||||
| Employee salaries and benefits payable |
513 | 484 | 208 | |||||||||
| 2,126 | 2,390 | 1,126 | ||||||||||
The Corporations exposure to currency and liquidity risks related to trade and other payables is presented in Note 19.
| 10. | Derivative warrant liabilities: |
Warrants issued as part of a public offering of units composed of class A share (Common Share) and Common Share purchase warrants in 2014 are derivative liabilities (Derivative warrant liabilities) for accounting purposes due to the currency of the exercise price being different from the Corporations functional currency.
The derivative warrant liabilities are measured at fair value at each reporting period and the reconciliation of changes in fair value is presented in the following table:
| Thirteen-month period | Month ended | Twelve-month period | Year ended | |||||||||||||
| ended March 31, 2017 | March 31, 2017
(Unaudited) |
ended February 28, 2017 (Unaudited) |
February 29, 2016 |
|||||||||||||
| $ | $ | $ | $ | |||||||||||||
| Balance beginning of period |
156 | 187 | 156 | 2,357 | ||||||||||||
| Change in fair value of derivative warrant liabilities |
53 | 22 | 31 | (2,201) | ||||||||||||
| Balance end of period |
209 | 209 | 187 | 156 | ||||||||||||
The fair value of the derivative warrant liabilities was estimated using the Black-Scholes option pricing model and based on the following assumptions:
| March 31, 2017 |
February 28, 2017 (Unaudited) |
February 29, 2016 | ||||||||||||||||||
| Exercise price |
US $1.50 | US $1.50 | US $1.50 | |||||||||||||||||
| Share price(1) |
US $1.36 | US $1.25 | US $1.50 | |||||||||||||||||
| Dividend |
| | | |||||||||||||||||
| Risk-free interest |
1.22% | 1.24% | 0.87% | |||||||||||||||||
| Estimated life |
1.68 years | 1.76 years | 2.76 years | |||||||||||||||||
| Expected volatility |
108.35% | 107.36% | 76.34% | |||||||||||||||||
| (1) | In order to obtain one Common Share, 10 warrants must be exercised. |
The fair value of the warrants issued was determined to be $0.11 per share issuable as at March 31, 2017 and $0.10 (unaudited) per share issuable as at February 28, 2017 ($0.09 per share issuable as at February 29, 2016).
F-41
Table of Contents
ACASTI PHARMA INC.
Notes to Financial Statements
Thirteen-month and one-month periods ended March 31, 2017, twelve-month period ended February 28, 2017 and years ended February 29, 2016 and February 28, 2015
(thousands of Canadian dollars, except where noted and for share and per share amounts)
| 11. | Unsecured convertible debentures |
Concurrent with the Public Offering described in note 12, on February 21, 2017, the Company issued $2,000 aggregate principal amount of unsecured convertible debentures maturing February 21, 2020 and contingent warrants to acquire up to 1,052,630 Common Shares (the Private Placement). The principal may be prepaid, in whole or in part, at any time and from time to time, in cash, at the sole discretion of the Corporation. The debentures are convertible into Common Shares at anytime by the holder at a fixed price of $1.90 per Common Share except if the Corporation pays before the maturity, all or any portion of the convertible debentures. Should the Corporation pay all or any portion of the convertible debenture before maturity, then warrants become exercisable at $1.90 per Common Share for the equivalent convertible debenture amount prepaid. The contingent warrants will be exercisable for the remaining term of the convertible debt for the same price as the conversion options. The unsecured convertible debentures were issued at a discount of 3.5% to the principal amount, for aggregate gross proceeds of $1,930.
The convertible debentures provide the Corporation an accelerated conversion right whereby the Corporation may, at any time at least four months after the date of issuance of the convertible debentures, accelerate the conversion of the debentures to Common Shares in the event that the volume weighted average price of the Corporations Common Shares on the TSX Venture Exchange is equal to or exceeds $2.65, subject to customary adjustment provisions, during 20 consecutive trading days.
The interest to be paid on the convertible debentures under the terms of the agreement is 8% per annum, payable on a quarterly basis in cash or Common Shares of the Corporation or a combination thereof, commencing on March 31, 2017. The decision to pay the interest due in cash or shares is at the discretion of the Corporation and the number of Common Shares to be issued will be calculated at the current market price as at the close of business on the day before the interest payment is to be made. Payment in shares shall be at a floor price of $0.10 per share, with the difference between the amount payable and the amount computed at floor price payable in cash.
The proceeds of the Private Placement were split between the liability and the equity at the time of issuance of the Private Placement. Both the conversion option and contingent warrants are considered the equity component of the Private Placement. The fair value of the liability component was determined through a discounted cash flow analysis using a discount rate of 20% that was set based on a similar debt and maturity considering the Corporations credit risk excluding the conversion option and contingent warrants. The amount allocated to the equity component is the residual amount after deducting the fair value of the financial liability component from the fair value of the entire compound instrument. Subsequent to initial recognition, the liability is measured at amortized cost calculated using the effective interest rate method and will accrete up to the principal balance at maturity. The interest accretion is presented as a financial expense. The equity component is not re-measured. Transaction costs were allocated to the components in proportion to their initial carrying amounts. The portion allocated to the liability was recognized as a reduction of the debt whereas the portion allocated to other equity was recognized as a reduction to other equity.
The fair value of the liability portion at the time of issuance was determined to be $1,519 and the transaction costs and debt discount amounted to $134, of which $30 is still unpaid as at March 31, 2017. The residual of the proceeds allocated to the equity component amounted to $481 and the transactions costs amounted to $43, of which $10 is unpaid at March 31, 2017.
F-42
Table of Contents
ACASTI PHARMA INC.
Notes to Financial Statements
Thirteen-month and one-month periods ended March 31, 2017, twelve-month period ended February 28, 2017 and years ended February 29, 2016 and February 28, 2015
(thousands of Canadian dollars, except where noted and for share and per share amounts)
| 11. | Unsecured convertible debentures (continued): |
The split between the liability and equity component portions of the Private Placement are summarized below:
| Liability component
|
Equity component | Total Private Placement | ||||
| $ | $ | $ | ||||
| Components at date of issue |
1,519 | 481 | 2,000 | |||
| Transaction costs and debt discount |
(134) | (43) | (177) | |||
| Deferred income tax expense (note 18) |
| (129) | (129) | |||
| Effective interest for the twelve-month period (Unaudited) |
8 | | 8 | |||
| Interest payable (Unaudited) |
(4) | | (4) | |||
| February 28, 2017 (Unaudited) |
1,389 | 309 | 1,698 | |||
| Effective interest for the one-month period (Unaudited) |
31 | | 31 | |||
| Interest payable (Unaudited) |
(14) | | (14) | |||
| Effective interest for the thirteen-month period |
39 | | 39 | |||
| Interest payable |
(18) | | (18) | |||
| March 31, 2017 |
1,406 | 309 | 1,715 | |||
| 12. | Capital and other components of equity |
| (a) | Share capital: |
Authorized capital stock:
Unlimited number of shares:
| Ø | Class A shares (Common Shares), voting (one vote per share), participating and without par value |
| Ø | Class B shares, voting (ten votes per share), non-participating, without par value and maximum annual non-cumulative dividend of 5% on the amount paid for said shares. Class B shares are convertible, at the holders discretion, into Class A shares (Common Shares), on a one-for-one basis, and Class B shares are redeemable at the holders discretion for $0.80 per share, subject to certain conditions. (1) |
| Ø | Class C shares, non-voting, non-participating, without par value and maximum annual non-cumulative dividend of 5% on the amount paid for said shares. Class C shares are convertible, at the holders discretion, into Class A shares (Common Shares), on a one-for-one basis, and Class C shares are redeemable at the holders discretion for $0.20 per share, subject to certain conditions. (1) |
| Ø | Class D and E shares, non-voting, non-participating, without par value and maximum monthly non-cumulative dividend between 0.5% and 2% on the amount paid for said shares. Class D and E shares are convertible, at the holders discretion, into Class A shares (Common Shares), on a one-for-one basis, and Class D and E shares are redeemable at the holders discretion, subject to certain conditions. (1) |
(1) None issued and outstanding
F-43
Table of Contents
ACASTI PHARMA INC.
Notes to Financial Statements
Thirteen-month and one-month periods ended March 31, 2017, twelve-month period ended February 28, 2017 and years ended February 29, 2016 and February 28, 2015
(thousands of Canadian dollars, except where noted and for share and per share amounts)
| 12. | Capital and other components of equity (continued): |
| (b) | Public offering 2017: |
Concurrent with the private placement described in Note 11, on February 21, 2017, the Corporation closed a public offering (Public Offering) issuing 3,930,518 units of Acasti (Units) at a price of $1.45 per Unit for gross proceeds of $5,699. Each Unit consists of one class A share (Common Share) and one half of one class A or common share purchase warrant. Each whole warrant entitles the holder thereof to purchase one common share at an exercise price of $2.15 per common share, at any time until February 21, 2022. The Units issued as part of the public offering are considered equity instruments. The transaction costs associated with the Public Offering amounted to $1,190, of which $381 remains unpaid as at March 31, 2017 (February 28, 2017 - $416 (unaudited)). The proceeds and transaction costs were allocated to share capital.
As part of the transaction, the Company also issued broker warrants (the Broker Warrants) to purchase up to 234,992 Common Shares. Each Broker Warrant entitles the holder thereof to acquire one Common Share of the Corporation at an exercise price of $2.15 per common share, at any time until February 21, 2018. The broker warrants are considered for compensation to non-employees under IFRS 2, stock-based compensation, and are accounted for at fair value through contributed surplus. To determine the fair value of the Broker Warrants, the Black-Scholes pricing model was used. The total costs associated with the Broker Warrants amounted to $144 and were allocated to share capital.
The warrants issued as part of the Units of the Public Offering and the broker warrants include an Acceleration Right, related to the Corporations right to accelerate the expiry date of the warrants. The Acceleration Right clause means the right of the Corporation to accelerate the expiry date to a date that is not less than 30 days following delivery of the acceleration notice if, at any time at least four months after the effective date, the volume weighted average trading price of the common shares equals or exceeds $2.65 for a period of 20 consecutive trading days on the TSXV.
Furthermore, as part of the February 2017 Public Offering and convertible debt transactions, a total of 60,000 Common Shares were issued as equity settled share-based payments for services received from an employee of the parent at a price of $1.57 per share for a total cost of $94. The equity settled share-based payment costs have been allocated to share capital for a cost that amounted to $85 and to debt for a cost that amounted to $9 based on relative value.
The value of the broker warrants was estimated using the Black-Scholes option pricing model and based on the following assumptions:
| Thirteen-month period ended March 31, 2017 |
||||
| Exercise price |
$2.15 | |||
| Share price |
$1.70 | |||
| Dividend |
| |||
| Risk-free interest |
0.79% | |||
| Estimated life |
1.00 year | |||
| Expected volatility |
112.09% | |||
| (c) | Issuance of shares: |
On February 5, 2016, 50,000 shares were issued on the settlement of a liability. An amount of $102, net of share issuance costs of $1, was recorded in share capital.
F-44
Table of Contents
ACASTI PHARMA INC.
Notes to Financial Statements
Thirteen-month and one-month periods ended March 31, 2017, twelve-month period ended February 28, 2017 and years ended February 29, 2016 and February 28, 2015
(thousands of Canadian dollars, except where noted and for share and per share amounts)
| 12. | Capital and other components of equity (continued): |
| (d) | Warrants: |
The warrants of the Corporation are composed of the following as at March 31, 2017, February 28, 2017, February 29, 2016 and February 28, 2015:
| March 31, 2017 |
February 28, (Unaudited) |
February 29, 2016 |
February 28, 2015 |
|||||||||||||||||||||||||||||
| Number outstanding | Amount | Number outstanding |
Amount | Number outstanding |
Amount | Number outstanding |
Amount | |||||||||||||||||||||||||
| $ | $ | $ | $ | |||||||||||||||||||||||||||||
| Liability |
||||||||||||||||||||||||||||||||
| Series 8 Public offering Warrants 2014 (note 10) (i) |
18,400,000 | 209 | 18,400,000 | 187 | 18,400,000 | 156 | 18,400,000 | 2,357 | ||||||||||||||||||||||||
| 18,400,000 | 209 | 18,400,000 | 187 | 18,400,000 | 156 | 18,400,000 | 2,357 | |||||||||||||||||||||||||
| Equity |
||||||||||||||||||||||||||||||||
| Public offering warrants |
||||||||||||||||||||||||||||||||
| Public offering warrants 2017 (ii) |
1,965,259 | | 1,965,259 | | | | | | ||||||||||||||||||||||||
| Series 2017-BW Broker warrants (iii) |
234,992 | 144 | 234,992 | 144 | | | | | ||||||||||||||||||||||||
| Private Placement contingent warrants |
||||||||||||||||||||||||||||||||
| 2017 Unsecured convertible debenture conversion option and contingent warrants (iv) |
1,052,630 | 309 | 1,052,630 | 309 | | | | | ||||||||||||||||||||||||
| Series 9 Private Placement warrants 2014 (v) |
161,654 | | 161,654 | | 161,654 | | 161,654 | | ||||||||||||||||||||||||
| 3,414,535 | 453 | 3,414,535 | 453 | 161,654 | | 161,654 | | |||||||||||||||||||||||||
| (i) | In order to obtain one Common Share of the Corporation at an exercise price of US$15.00, 10 warrants must be exercised. Warrants expire on December 3, 2018. |
| (ii) | Warrant to acquire one Common Share of the Corporation at an exercise price of $2.15, expiring on February 21, 2022. |
| (iii) | Warrant to acquire one Common Share of the Corporation at an exercise price of $2.15 expiring on February 21, 2018. |
| (iv) | Warrant to acquire one Common Share of the Corporation at an exercise price of $1.90 expiring on February 21, 2020, net of deferred tax expense of $129. |
| (v) | Warrant to acquire one Common Share of the Corporation at an exercise price of $13.30, expiring on December 3, 2018. |
F-45
Table of Contents
ACASTI PHARMA INC.
Notes to Financial Statements
Thirteen-month and one-month periods ended March 31, 2017, twelve-month period ended February 28, 2017 and years ended February 29, 2016 and February 28, 2015
(thousands of Canadian dollars, except where noted and for share and per share amounts)
| 13. | Personnel expenses: |
|
Thirteen-months ended |
Month ended | Twelve-month period ended |
Year ended | Year ended | ||||||||||||||||
| March 31, 2017 |
March 31, 2017 (Unaudited) |
February 28, (Unaudited) |
February 29, 2016 |
February 28, 2015 |
||||||||||||||||
|
|
$ | $ | $ | $ | $ | |||||||||||||||
| Salaries and other short-term employee benefits |
2,483 | 214 | 2,269 | 1,902 | 1,554 | |||||||||||||||
| Share-based compensation costs |
674 | 86 | 588 | 309 | 1,553 | |||||||||||||||
| Severance |
| | | 210 | 171 | |||||||||||||||
| 3,157 | 300 | 2,857 | 2,421 | 3,278 | ||||||||||||||||
| 14. | Financial (expenses) income: |
|
Thirteen-months ended |
Month ended | Twelve-month period ended |
Year ended | Year ended | ||||||||||||||||
| March 31, 2017 |
March 31, 2017 (Unaudited) |
February 28, (Unaudited) |
February 29, 2016 |
February 28, 2015 |
||||||||||||||||
| $ | $ | $ | $ | $ | ||||||||||||||||
| Interest income |
125 | 6 | 119 | 73 | 87 | |||||||||||||||
| Foreign exchange gain |
| | | 1,023 | 1,833 | |||||||||||||||
| Financial income |
125 | 6 | 119 | 1,096 | 1,920 | |||||||||||||||
| Foreign exchange loss |
(180) | (3) | (177) | | | |||||||||||||||
| Interest on convertible debenture |
(39) | (31) | (8) | | | |||||||||||||||
| Other charges |
(19) | (1) | (18) | (2) | (4) | |||||||||||||||
| Financial expenses |
(238) | (35) | (203) | (2) | (4) | |||||||||||||||
| Financial (expenses) income |
(113) | (29) | (84) | 1,094 | 1,916 | |||||||||||||||
F-46
Table of Contents
ACASTI PHARMA INC.
Notes to Financial Statements
Thirteen-month and one-month periods ended March 31, 2017, twelve-month period ended February 28, 2017 and years ended February 29, 2016 and February 28, 2015
(thousands of Canadian dollars, except where noted and for share and per share amounts)
| 15. | Share-based payments: |
At March 31, 2017, the Corporation has the following share-based payment arrangement:
| (a) | Corporation stock option plan: |
The Corporation has in place a stock option plan for directors, officers, employees and consultants of the Corporation. The plan provides for the granting of options to purchase Class A shares (Common Shares). The exercise price of the stock options granted under this plan is not lower than the closing price of the shares listed on the TSXV at the close of markets the day preceding the grant. Under this plan, the maximum number of Class A shares (Common Shares) that may be issued upon exercise of options granted under the plan is 2,142,407, representing 20% of the number of Class A shares (Common Shares) issued and outstanding as at February 29, 2016. The terms and conditions for acquiring and exercising options are set by the Corporations Board of Directors, subject among others, to the following limitations: the term of the options cannot exceed ten years and every stock option granted under the stock option plan will be subject to conditions no less restrictive than a minimum vesting period of 18 months and a gradual and equal acquisition of vesting rights not shorter than on a quarterly basis. The total number of shares issued to any one consultant cannot exceed 2% of the Corporations total issued and outstanding shares. The Corporation is not authorized to grant such number of options under the stock option plan that could result in a number of Class A shares (Common Shares) issuable pursuant to options granted to (a) related persons exceeding 10% of the Corporations issued and outstanding Class A shares (Common Shares) (on a non-diluted basis) on the date an option is granted, or (b) any one eligible person in a twelve month period exceeding 5% of the Corporations issued and outstanding Class A shares (Common Shares) (on a non-diluted basis) on the date an option is granted.
The following tables summarize information about activities within the stock option plan:
|
|
||||||||
| Thirteen-month period ended | ||||||||
| March 31, 2017 | ||||||||
|
|
|
|||||||
| Weighted average exercise price |
Number of options |
|||||||
|
|
||||||||
| $ | ||||||||
|
|
||||||||
| Outstanding at beginning of period |
13.52 | 454,151 | ||||||
| Granted |
1.69 | 1,300,400 | ||||||
| Forfeited |
13.27 | (190,138 | ) | |||||
| Expired |
15.38 | (139,625 | ) | |||||
|
|
||||||||
| Outstanding at end of period |
2.58 | 1,424,788 | ||||||
|
|
||||||||
| Exercisable at end of period |
6.44 | 238,482 | ||||||
|
|
||||||||
|
|
||||||||
| Month ended | Twelve-month period ended | |||||||||||||||
| March 31, 2017 (Unaudited) |
February 28, 2017 (Unaudited) |
|||||||||||||||
|
|
|
|
|
|||||||||||||
| Weighted average exercise price |
Number of options |
Weighted average exercise price |
Number of options |
|||||||||||||
|
|
||||||||||||||||
| $ | $ | |||||||||||||||
|
|
||||||||||||||||
| Outstanding at beginning of period |
2.59 | 1,427,288 | 13.52 | 454,151 | ||||||||||||
| Granted |
| | 1.69 | 1,300,400 | ||||||||||||
| Forfeited |
11.50 | (2,500 | ) | 13.29 | (187,638 | ) | ||||||||||
| Expired |
| | 15.38 | (139,625 | ) | |||||||||||
|
|
||||||||||||||||
| Outstanding at end of period |
2.58 | 1,424,788 | 2.59 | 1,427,288 | ||||||||||||
|
|
||||||||||||||||
| Exercisable at end of period |
6.44 | 238,482 | 6.49 | 240,982 | ||||||||||||
|
|
||||||||||||||||
F-47
Table of Contents
ACASTI PHARMA INC.
Notes to Financial Statements
Thirteen-month and one-month periods ended March 31, 2017, twelve-month period ended February 28, 2017 and years ended February 29, 2016 and February 28, 2015
(thousands of Canadian dollars, except where noted and for share and per share amounts)
| 15. | Share-based payments (continued): |
| (a) | Corporation stock option plan (continued): |
|
|
||||||||||||||||
| Year ended | Year ended | |||||||||||||||
| February 29, 2016 | February 28, 2015 | |||||||||||||||
|
|
|
|
|
|||||||||||||
| Weighted average exercise price |
Number of options |
Weighted average exercise price |
Number of options |
|||||||||||||
|
|
||||||||||||||||
| $ | $ | |||||||||||||||
|
|
||||||||||||||||
| Outstanding at beginning of year |
15.33 | 429,625 | 15.72 | 491,100 | ||||||||||||
| Granted |
4.65 | 109,188 | 9.51 | 51,250 | ||||||||||||
| Exercised |
2.50 | (250 | ) | 2.50 | (20,000 | ) | ||||||||||
| Forfeited |
9.40 | (66,912 | ) | 14.90 | (22,725 | ) | ||||||||||
| Expired |
18.57 | (17,500 | ) | 18.00 | (10,000 | ) | ||||||||||
| Cancelled |
| | 17.50 | (60,000 | ) | |||||||||||
|
|
||||||||||||||||
| Outstanding at end of year |
13.52 | 454,151 | 15.33 | 429,625 | ||||||||||||
|
|
||||||||||||||||
| Exercisable at end of year |
15.28 | 375,563 | 15.48 | 332,039 | ||||||||||||
|
|
||||||||||||||||
The weighted average of the fair value of the options granted to employees and directors of the Company during the thirteen-month period ended March 31, 2017 is $1.40 and during the twelve-month period ended February 28, 2017 is $1.40 (unaudited) (2016 -$2.14 and 2015 - $3.52). There were no options granted during the month ended March 31, 2017 and no options granted to consultants during the thirteen-month period ended March 31, 2017 and years ended February 29, 2016 and February 28, 2015.
No options were exercised during the thirteen-month period ended March 31, 2017. The weighted average share price at the date of exercise for share options exercised during the year ended February 29, 2016 was $4.20 (2015 - $9.20). Stock-based compensation recognized under this plan for the thirteen-month and one-month periods ended March 31, 2017 amounted to $674 and $86 (unaudited), respectively and amounted to $588 (unaudited) for the twelve-month period ended February 28, 2017 (2016 - $234 and 2015 - $526).
The fair value of options granted was estimated using the Black-Scholes option pricing model, resulting in the following weighted average assumptions for options granted during the periods ended:
|
Thirteen-month period ended |
Twelve-month Period ended |
Year ended | Year ended | |||||||||||||
| March 31, 2017 |
February 28, 2017 (Unaudited) |
February 29, 2016 | February 28, 2015 | |||||||||||||
| Exercise price |
$1.69 | $1.69 | $4.65 | $9.51 | ||||||||||||
| Share price |
$1.69 | $1.69 | $4.65 | $9.51 | ||||||||||||
| Dividend |
| | | | ||||||||||||
| Risk-free interest |
0.87% | 0.87% | 0.66% | 1.14% | ||||||||||||
| Estimated life |
4.94 years | 4.94 years | 4.20 years | 3.00 years | ||||||||||||
| Expected volatility |
123.54% | 123.54% | 65.63% | 60.34% | ||||||||||||
The expected life of the stock options is based on historical data and current expectation and is not necessarily indicative of exercise patterns that may occur. The expected volatility reflects the assumption that the historical volatility over a period similar to the life of the options is indicative of future trends, which may also not necessarily be the actual outcome.
F-48
Table of Contents
ACASTI PHARMA INC.
Notes to Financial Statements
Thirteen-month and one-month periods ended March 31, 2017, twelve-month period ended February 28, 2017 and years ended February 29, 2016 and February 28, 2015
(thousands of Canadian dollars, except where noted and for share and per share amounts)
| 15. | Share-based payments (continued): |
| (a) | Corporation stock option plan (continued): |
The following tables summarize the status of the outstanding and exercisable options of the Corporation:
| March 31, 2017 | ||||||||||||||||||||
| Options outstanding | Exercisable options | |||||||||||||||||||
| Exercise price | Weighted remaining contractual life |
Number of options |
Weighed average exercise price $ |
Number of options |
||||||||||||||||
| $1.56 - $1.61 |
6.11 | 525,000 | 1.56 | 131,250 | ||||||||||||||||
| $1.62 - $1.82 |
9.90 | 465,000 | | | ||||||||||||||||
| $1.83 - $2.25 |
6.16 | 286,700 | | | ||||||||||||||||
| $2.26 - $5.65 |
4.08 | 79,588 | 3.84 | 38,732 | ||||||||||||||||
| $5.66 - $21.00 |
0.64 | 68,500 | 17.26 | 68,500 | ||||||||||||||||
| 6.98 | 1,424,788 | 6.44 | 238,482 | |||||||||||||||||
| February 28, 2017 (Unaudited) | ||||||||||||||||||||
| Options outstanding | Exercisable options | |||||||||||||||||||
| Exercise price | Weighted remaining contractual life Outstanding |
Number of options Outstanding |
Weighed average |
Number of options exercisable |
||||||||||||||||
| $1.56 - $1.61 |
6.20 | 525,000 | 1.56 | 131,250 | ||||||||||||||||
| $1.62 - $1.82 |
9.99 | 465,000 | | | ||||||||||||||||
| $1.83 - $2.25 |
6.25 | 286,700 | | | ||||||||||||||||
| $2.26 - $5.65 |
4.17 | 79,588 | 3.84 | 38,732 | ||||||||||||||||
| $5.66 - $21.00 |
0.71 | 71,000 | 17.06 | 71,000 | ||||||||||||||||
| 7.06 | 1,427,288 | 6.49 | 240,982 | |||||||||||||||||
Share-based payment transactions and broker warrants:
The fair value of share-based payment transaction is measured using the Black-Scholes valuation model. Measurement inputs include share price on measurement date, exercise price of the instrument, expected volatility (based on weighted average historic volatility), weighted average expected life of the instruments (based on historical experience and general option holder behaviour unless no entity-specific information exists in which case the average of the vesting and contractual periods is used), expected dividends, and the risk-free interest rate (based on government bonds). Service and non-market performance conditions attached to the transactions, if any, are not taken into account in determining fair value.
| b) | Corporation equity incentive plan: |
The Corporation established an equity incentive plan for employees, directors and consultants. The plan provides for the issuance of restricted share units (RSU), performance share units, restricted shares, deferred share units and other share-based awards, subject to restricted conditions as may be determined by the Board of Directors. There are no such awards outstanding as of March 31, 2017, February 28, 2017 and February 29, 2016 and no stock-based compensation was recognized for the one-month and thirteen-month periods ended March 31, 2017 and $64 for the twelve-month period ended February 29, 2016 (2015 - $466).
F-49
Table of Contents
ACASTI PHARMA INC.
Notes to Financial Statements
Thirteen-month and one-month periods ended March 31, 2017, twelve-month period ended February 28, 2017 and years ended February 29, 2016 and February 28, 2015
(thousands of Canadian dollars, except where noted and for share and per share amounts)
| 16. | Loss per share: |
Diluted loss per share was the same amount as basic loss per share, as the effect of options, RSUs and warrants would have been anti-dilutive, because the Corporation incurred losses in each of the periods presented. All outstanding options, RSUs and warrants could potentially be dilutive in the future.
| 17. | Supplemental cash flow disclosure: |
| (a) | Changes in non-cash operating items: |
|
Thirteen-months ended |
Month ended | Twelve-months ended |
Year ended | Year ended | ||||||||||||||||
| March 31, 2017 |
March 31, 2017 (Unaudited) |
February 28, (Unaudited) |
February 29, 2016 |
February 28, 2015 |
||||||||||||||||
| $ | $ | $ | $ | $ | ||||||||||||||||
| Receivables |
193 | (40) | 233 | 406 | 248 | |||||||||||||||
| Receivable from corporation under common control |
| | | 50 | 47 | |||||||||||||||
| Inventories |
| | | 88 | 174 | |||||||||||||||
| Prepaid expenses |
247 | (33) | 280 | (138) | 385 | |||||||||||||||
| Trade and other payables |
382 | (252) | 634 | 50 | (87) | |||||||||||||||
| Receivable/payable to parent corporation |
(30) | (3) | (27) | (497) | 539 | |||||||||||||||
| 792 | (328) | 1,120 | (41) | 1,306 | ||||||||||||||||
|
(b) Non-cash transactions:
|
||||||||||||||||||||
| Thirteen-months ended |
Month ended | Twelve-months ended |
Year ended | Year ended | ||||||||||||||||
| March 31, 2017 |
March 31, 2017 (Unaudited) |
February 28, (Unaudited) |
February 29, 2016 |
February 28, 2015 |
||||||||||||||||
| $ | $ | $ | $ | $ | ||||||||||||||||
| Equity settled share-based payment included in equity ($85) and unsecured convertible debentures ($9) |
94 | | 94 | | | |||||||||||||||
| Issuance of broker warrants included in net proceeds from public offering |
144 | | 144 | | | |||||||||||||||
| Public offering transaction costs included in trade and other payables |
381 | 381 | 416 | | | |||||||||||||||
| Reduction in share issue costs from reduction in trade and other payables |
109 | | 109 | | | |||||||||||||||
| Private Placement transaction costs included in trade and other payables |
40 | 40 | 50 | | | |||||||||||||||
| Equipment included in trade and other payables |
288 | 288 | 269 | | | |||||||||||||||
| Interest payable included in trade and other payables |
18 | 18 | 4 | | | |||||||||||||||
| Issuance of shares on settlement of a liability |
| | | 103 | | |||||||||||||||
| Intangible assets included in trade and other payables |
| | | | 8 | |||||||||||||||
| Interest receivable included in payable to parent corporation |
| | | 27 | | |||||||||||||||
F-50
Table of Contents
ACASTI PHARMA INC.
Notes to Financial Statements
Thirteen-month and one-month periods ended March 31, 2017, twelve-month period ended February 28, 2017 and years ended February 29, 2016 and February 28, 2015
(thousands of Canadian dollars, except where noted and for share and per share amounts)
| 18. | Income taxes: |
Deferred tax (recovery) expense:
| Thirteen-months ended |
Month ended |
Twelve-months ended |
Year ended | Year ended | ||||||||||||||||
| March 31, 2017 |
March 31, 2017 (Unaudited) |
February 28, 2017 (Unaudited) |
February 29, 2016 |
February 28, 2015 |
||||||||||||||||
| $ | $ | $ | $ | $ | ||||||||||||||||
| Origination and reversal of temporary differences |
2,240 | 163 | 2,077 | 2,065 | 2,221 | |||||||||||||||
| Change in unrecognized deductible temporary differences |
(2,369) | (163) | (2,206) | (2,065) | (2,221) | |||||||||||||||
| Deferred tax (recovery) expense |
(129) | | (129) | | | |||||||||||||||
|
Reconciliation of effective tax rate:
|
||||||||||||||||||||
| Thirteen-months ended |
Month ended |
Twelve-months ended |
Year ended | Year ended | ||||||||||||||||
| March 31, 2017 |
March 31, 2017 (Unaudited) |
February 28, 2017 (Unaudited) |
February 29, 2016 |
February 28, 2015 |
||||||||||||||||
| $ | $ | $ | $ | $ | ||||||||||||||||
| Loss before income taxes |
(11,376) | (769) | (10,607) | (6,317) | (1,654) | |||||||||||||||
| Basic combined Canadian statutory income tax rate 1 |
26.87% | 26.80% | 26.88% | 26.90% | 26.90% | |||||||||||||||
| Computed income tax recovery |
(3,057) | (207) | (2,850) | (1,699) | (445) | |||||||||||||||
| Increase resulting from: |
||||||||||||||||||||
| Change in unrecognized deductible temporary differences |
2,369 | 163 | 2,206 | 2,065 | 2,221 | |||||||||||||||
| Non-deductible stock-based compensation |
178 | 23 | 155 | 83 | 418 | |||||||||||||||
| Non-deductible change in fair value |
14 | 6 | 8 | (592) | (2,374) | |||||||||||||||
| Permanent differences and other |
166 | 12 | 154 | 143 | 180 | |||||||||||||||
| Change in statutory income tax rate |
201 | 3 | 198 | | | |||||||||||||||
| Total tax (recovery) expense |
(129) | | (129) | | | |||||||||||||||
1 The Canadian combined statutory income tax rate has decreased due to a reduction in the provincial statutory income tax rate.
F-51
Table of Contents
ACASTI PHARMA INC.
Notes to Financial Statements
Thirteen-month and one-month periods ended March 31, 2017, twelve-month period ended February 28, 2017 and years ended February 29, 2016 and February 28, 2015
(thousands of Canadian dollars, except where noted and for share and per share amounts)
| 18. | Income taxes (continued): |
Unrecognized deferred tax assets:
At March 31, 2017, February 28, 2017 and February 29, 2016, the net deferred tax assets, which have not been recognized in these financial statements because the criteria for recognition of these assets were not met, were as follows:
| March 31, 2017 | February 28, 2017 | February 29, 2016 | ||||||||||
|
|
(Unaudited) | |||||||||||
| $ | $ | $ | ||||||||||
| Deferred tax assets |
||||||||||||
| Tax losses carried forward |
8,293 | 8,153 | 6,020 | |||||||||
| Research and development expenses |
4,220 | 4,196 | 3,866 | |||||||||
| Property, plan and equipment and intangible assets |
435 | 423 | 340 | |||||||||
| Other deductible temporary differences |
522 | 539 | 388 | |||||||||
| Deferred tax assets |
13,470 | 13,311 | 10,614 | |||||||||
| Deferred tax liabilities |
||||||||||||
| Tax basis of unsecured convertible debentures in excess of carrying value |
122 | 126 | | |||||||||
| Deferred tax liabilities |
122 | 126 | | |||||||||
| Net deferred tax assets |
13,348 | 13,185 | 10,614 | |||||||||
On initial recognition of the unsecured convertible debenture equity component, a deferred tax liability of $129 was recognized with the corresponding entry recognized directly in Other equity. Consequently, an equal amount of deferred tax asset related to unrecognized tax losses was recognized with the offsetting entry in the Corporation statement of earnings and comprehensive loss.
As at March 31, 2017 and February 28, 2017, the amounts and expiry dates of tax attributes and temporary differences, which are available to reduce future years taxable income, were as follows:
| March 31, 2017 | February 28, 2017 | |||||||||||||||
| (Unaudited) | ||||||||||||||||
| Federal | Provincial | Federal | Provincial | |||||||||||||
| $ | $ | $ | $ | |||||||||||||
| Tax losses carried forward |
||||||||||||||||
| 2029 |
714 | 714 | 714 | 714 | ||||||||||||
| 2030 |
1,627 | 1,620 | 1,627 | 1,620 | ||||||||||||
| 2031 |
2,071 | 2,063 | 2,071 | 2,063 | ||||||||||||
| 2032 |
2,262 | 2,241 | 2,262 | 2,241 | ||||||||||||
| 2033 |
1,854 | 1,825 | 1,854 | 1,825 | ||||||||||||
| 2034 |
3,597 | 3,597 | 3,597 | 3,597 | ||||||||||||
| 2035 |
4,595 | 4,595 | 4,595 | 4,595 | ||||||||||||
| 2036 |
5,494 | 5,494 | 5,494 | 5,494 | ||||||||||||
| 2037 |
9,109 | 9,109 | 8,579 | 8,579 | ||||||||||||
| 31,323 | 31,260 | 30,793 | 30,728 | |||||||||||||
| Research and development expenses, without time limitation |
15,436 | 16,559 | 15,347 | 16,469 | ||||||||||||
| Other deductible temporary differences, without time limitation |
3,154 | 3,154 | 3,158 | 3,158 | ||||||||||||
F-52
Table of Contents
ACASTI PHARMA INC.
Notes to Financial Statements
Thirteen-month and one-month periods ended March 31, 2017, twelve-month period ended February 28, 2017 and years ended February 29, 2016 and February 28, 2015
(thousands of Canadian dollars, except where noted and for share and per share amounts)
| 19. | Financial instruments: |
This note provides disclosures relating to the nature and extent of the Corporations exposure to risks arising from financial instruments, including credit risk, foreign currency risk, interest rate risk and liquidity risk, and how the Corporation manages those risks.
| (a) | Credit risk: |
Credit risk is the risk of a loss if a customer or counterparty to a financial asset fails to meet its contractual obligations. The Corporation has credit risk relating to cash and cash equivalents and short-term investments, which it manages by dealing only with highly-rated Canadian institutions. The carrying amount of financial assets, as disclosed in the statements of financial position, represents the Corporations credit exposure at the reporting date.
| (b) | Currency risk: |
The Corporation is exposed to the financial risk related to the fluctuation of foreign exchange rates and the degrees of volatility of those rates. Foreign currency risk is limited to the portion of the Corporations business transactions denominated in currencies other than the Canadian dollar. Fluctuations related to foreign exchange rates could cause unforeseen fluctuations in the Corporations operating results.
A portion of the expenses, mainly related to research contracts and purchase of production equipment, is incurred in US dollars and in Euros, for which no financial hedging is required. There is a financial risk related to the fluctuation in the value of the US dollar and the Euro in relation to the Canadian dollar. In order to minimize the financial risk related to the fluctuation in the value of the US dollar in relation to the Canadian dollar, funds continue to be invested as short-term investments in the US dollar.
The following table provides an indication of the Corporations significant foreign exchange currency exposures as stated in Canadian dollars at the following dates:
| March 31, 2017 |
February 28, 2017 (Unaudited) |
February 29, 2016 |
||||||||||||||||||
| US$ | Euro | US$ | Euro | US$ | ||||||||||||||||
| Cash and cash equivalents |
3,524 | | 3,691 | | 2,872 | |||||||||||||||
| Short-term investments |
| | | | 7,442 | |||||||||||||||
| Receivables |
2 | | 3 | | 1 | |||||||||||||||
| Trade and other payables |
(503) | (317) | (376) | (603) | (275) | |||||||||||||||
| 3,023 | (317) | 3,318 | (603) | 10,040 | ||||||||||||||||
The following exchange rates are those applicable to the following periods and dates:
| March 31, 2017 |
February 28, 2017 |
February 29, 2016 |
||||||||||||||||||||||
| (Unaudited) | ||||||||||||||||||||||||
| Average | Reporting | Average | Reporting | Average | Reporting | |||||||||||||||||||
| CA$ per US$ |
1.3134 | 1.3299 | 1.3113 | 1.3281 | 1.3071 | 1.3531 | ||||||||||||||||||
| CA$ per Euro |
1.4424 | 1.4251 | 1.4434 | 1.4066 | 1.4393 | | ||||||||||||||||||
F-53
Table of Contents
ACASTI PHARMA INC.
Notes to Financial Statements
Thirteen-month and one-month periods ended March 31, 2017, twelve-month period ended February 28, 2017 and years ended February 29, 2016 and February 28, 2015
(thousands of Canadian dollars, except where noted and for share and per share amounts)
| 19. | Financial instruments (continued): |
| (b) | Currency risk (continued): |
Based on the Corporations foreign currency exposures noted above, varying the above foreign exchange rates to reflect a 5% strengthening of the US dollar and Euro would have decrease in net loss as follows, assuming that all other variables remain constant:
| March 31, 2017 | February 28, 2017 | February 29, 2016 | ||||||||||
| (Unaudited) | ||||||||||||
| $ | $ | $ | ||||||||||
| Decrease in net loss |
139 | 151 | 502 | |||||||||
An assumed 5% weakening of the foreign currencies would have an equal but opposite effect on the basis that all other variables remained constant.
| (c) | Interest rate risk: |
Interest rate risk is the risk that the fair value or future cash flows of a financial instrument will fluctuate because of changes in market rates.
The Corporations exposure to interest rate risk as at March 31, 2017, February 28, 2017 and February 29, 2016 is as follows:
| Cash and cash equivalents |
Short-term fixed interest rate | |||
| Short-term investments |
Short-term fixed interest rate | |||
| Unsecured convertible debentures |
Long-term fixed interest rate | |||
The capacity of the Corporation to reinvest the short-term amounts with equivalent return will be impacted by variations in short-term fixed interest rates available on the market. Management believes that the risk the Corporation will realize a loss as a result of the decline in the fair value of its cash equivalents is limited because these investments have short-term maturities and are generally held to maturity.
| (d) | Liquidity risk: |
Liquidity risk is the risk that the Corporation will not be able to meet its financial obligations as they fall due. The Corporation manages liquidity risk through the management of its capital structure and financial leverage, as outlined in Note 22. It also manages liquidity risk by continuously monitoring actual and projected cash flows. The Board of Directors reviews and approves the Corporations operating budgets, and reviews material transactions outside the normal course of business. Refer to Note 2(c).
F-54
Table of Contents
ACASTI PHARMA INC.
Notes to Financial Statements
Thirteen-month and one-month periods ended March 31, 2017, twelve-month period ended February 28, 2017 and years ended February 29, 2016 and February 28, 2015
(thousands of Canadian dollars, except where noted and for share and per share amounts)
| 19. | Financial instruments (continued): |
| (d) | Liquidity risk (continued): |
The following are the contractual maturities of financial liabilities as at March 31, 2017, February 28, 2017 and February 29, 2016:
| March 31, 2017 | ||||||||||||||||||
| Required payments per year | Notes | Total $ |
Carrying amount $ |
Less than 1 year $ |
1 to 3 years $ |
|||||||||||||
| Trade and other payables |
9 | 2,126 | 2,126 | 2,126 | | |||||||||||||
| Payable to parent corporation |
5(c) | 12 | 12 | 12 | | |||||||||||||
| Unsecured convertible debentures |
11 | 2,463 | 1,406 | 160 | 2,303 | |||||||||||||
| 4,601 | 3,544 | 2,298 | 2,303 | |||||||||||||||
| February 28, 2017 (Unaudited) |
||||||||||||||||||
| Required payments per year | Total $ |
Carrying amount $ |
Less than 1 year $ |
1 to 3 years $ |
||||||||||||||
| Trade and other payables |
9 | 2,390 | 2,390 | 2,390 | | |||||||||||||
| Payable to parent corporation |
5(c) | 15 | 15 | 15 | | |||||||||||||
| Unsecured convertible debentures |
11 | 2,480 | 1,389 | 160 | 2,316 | |||||||||||||
| 4,885 | 3,794 | 2,565 | 2,316 | |||||||||||||||
| February 29, 2016 | ||||||||||||||||||
| Required payments per year | Total $ |
Carrying amount $ |
Less than 1 year $ |
1 to 3 years $ |
||||||||||||||
| Trade and other payables |
9 | 1,126 | 1,126 | 1,126 | | |||||||||||||
| Payable to parent corporation |
5(c) | 15 | 15 | 15 | | |||||||||||||
| 1,141 | 1,141 | 1,141 | | |||||||||||||||
The Derivative warrant liabilities are excluded from the above tables as they will be settled in shares and not by the use of liquidities.
F-55
Table of Contents
ACASTI PHARMA INC.
Notes to Financial Statements
Thirteen-month and one-month periods ended March 31, 2017, twelve-month period ended February 28, 2017 and years ended February 29, 2016 and February 28, 2015
(thousands of Canadian dollars, except where noted and for share and per share amounts)
| 20. | Commitments and contingencies: |
Research and development agreements:
In the normal course of business, the Corporation has signed agreements with various partners and suppliers for them to execute research and development projects and to produce certain tools and equipment. The Corporation has reserved certain rights relating to these projects.
The Corporation initiated research and development projects that are planned to be conducted over the next 12-month period for a total cost of $2,169 of which an amount of $785 has been paid to date. As at March 31, 2017, an amount of $467 is included in Trade and other payables in relation to these projects.
The Corporation has also entered into a contract to purchase production equipment for a total cost of $1,162 to be used in the manufacturing of the clinical and future commercial supply of CaPre®, of which an amount of $853 has been paid to date. As at March 31, 2017, an amount of $288 is included in Trade and other payables related to this equipment.
Contingencies:
A former CEO of the Corporation is claiming the payment of approximately $8.5 million and the issuance of equity instruments from the Group. As the Corporations management believes that these claims are not valid, no provision has been recognized. Neptune and its subsidiaries also filed an additional claim to recover certain amounts from the former officer. All outstanding share-based payments held by the former CEO have been cancelled during the year ended February 28, 2015.
The Corporation is also involved in other matters arising in the ordinary course of its business. Since management believes that all related claims are not valid and it is presently not possible to determine the outcome of these matters, no provisions have been made in the financial statements for their ultimate resolution beyond the amounts incurred and recorded for such matters. The resolution of such matters could have an effect on the Corporations financial statements in the year that a determination is made, however, in managements opinion, the final resolution of all such matters is not projected to have a material adverse effect on the Corporations financial position.
| 21. | Determination of fair values: |
Certain of the Corporations accounting policies and disclosures require the determination of fair value, for both financial assets and liabilities. Fair values have been determined for measurement and/or disclosure purposes based on the following methods.
Financial assets and liabilities:
In establishing fair value, the Corporation uses a fair value hierarchy based on levels as defined below:
| | Level 1: defined as observable inputs such as quoted prices in active markets. |
| | Level 2: defined as inputs other than quoted prices in active markets that are either directly or indirectly observable. |
| | Level 3: defined as inputs that are based on little or no observable market data, therefore requiring entities to develop their own assumptions. |
The Corporation has determined that the carrying values of its short-term financial assets and liabilities approximate their fair value given the short-term nature of these instruments. The fair value of the liability component of the convertible debenture is determined by discounting future cash flows using a rate that the Corporation could obtain for loans with similar terms, conditions and maturity dates. The fair value of this liability at February 28, 2017 and March 31, 2017 has not changed from the issuance date of February 21, 2017 and was measured using level 3 inputs.
F-56
Table of Contents
ACASTI PHARMA INC.
Notes to Financial Statements
Thirteen-month and one-month periods ended March 31, 2017, twelve-month period ended February 28, 2017 and years ended February 29, 2016 and February 28, 2015
(thousands of Canadian dollars, except where noted and for share and per share amounts)
| 21. | Determination of fair values (continued): |
Derivative warrant liabilities:
The Corporation measured its derivative warrant liabilities at fair value on a recurring basis. These financial liabilities were measured using a level 3 inputs (Note 10).
As at March 31, 2017, the effect of an increase or a decrease of 5% of the volatility used, which is the significant unobservable input in the fair value estimate, would result in a loss of $49 or a gain of $44, respectively. As at February 28, 2017, the effect of an increase or a decrease of 5% of the volatility used, which is the significant unobservable input in the fair value estimate, would result in a loss of $45 (unaudited) or a gain of $40 (unaudited), respectively.
| 22. | Capital management: |
Since inception, the Corporations objective in managing capital is to ensure sufficient liquidity to finance its research and development activities, general and administrative expenses, expenses associated with intellectual property protection and its overall capital expenditures. The Corporation is not exposed to external requirements by regulatory agencies or third parties regarding its capital, except for certain covenants included within the convertible debentures (Note 11).
Since the beginning of its operations, the Corporation has primarily financed its liquidity needs from funding provided through public offerings, private placements, its parent corporation, from the exercise of warrants that were distributed to its parent corporations shareholders, from a rights offering and from the issuance of options to employees. However, the Corporation attempts to optimize its liquidity needs with non-dilutive sources whenever possible, including from research and development tax credits or government assistance.
The Corporation defines capital to include total shareholders equity, derivative warrant liabilities and unsecured convertible debentures.
The Corporations policy is to maintain a minimal level of debt.
The following table summarizes the cash and cash equivalents and short-term investments of the Corporation:
| March 31, 2017 | February 28, 2017 (Unaudited) |
February 29, 2016 | ||||||||||
| Cash |
6,778 | 7,584 | 3,027 | |||||||||
| Cash equivalents |
2,994 | 2,989 | | |||||||||
| Short-term investments |
| | 7,443 | |||||||||
| 9,772 | 10,573 | 10,470 | ||||||||||
As at March 31, 2017 and February 28, 2017, cash equivalents consisting of two term deposits totaling $2,994 (US - $2,251) and $2,990 (US$2,251) (unaudited), respectively, are being held with a Canadian financial institution having a high credit rating. The term deposits as at March 31, 2017 have maturity dates of April 11, 2017 and April 25, 2017, bearing an interest rate of 0.52% and 0.53% per annum, respectively, cashable at any time at the discretion of the Corporation, under certain conditions. The term deposits as at February 28, 2017 have maturity dates of March 12, 2017 and March 28, 2017, bearing an interest rate of 0.46% and 0.45% per annum, respectively, cashable at any time at the discretion of the Corporation, under certain conditions.
As at February 29, 2016, a short-term investment consisting of a term deposit totaling $7,443 (US - $5,500) was with a Canadian financial institution having a high credit rating. The short-term investment had a maturity date of March 29, 2016, bearing an interest rate of 0.33% per annum, cashable at any time at the discretion of the Corporation, under certain conditions.
F-57
Table of Contents

Acasti Pharma Inc.
9,900,990 Common Shares and Warrants
to Purchase up to 8,910,891 Common Shares
(8,910,891 Common Shares Underlying the Warrants)
PROSPECTUS
| Benchmark | Dawson James Securities, Inc. |
December 21, 2017