FWP: Filing under Securities Act Rules 163/433 of free writing prospectuses
Published on November 24, 2017

NASDAQ & TSX-V: ACST Company Overview November/December 2017 Issuer Free Writing Prospectus Filed Pursuant to Rule 433 of the Securities Act of 1933, as amended Registration Statement No. 333-220755 COMPANY OVERVIEW

Notices This presentation contains information that may be forward-looking statements within the meaning of applicable securities laws. Forward-looking statements can be identified by the use of terms such as “may”, “will”, “should”, “expect”, “plan”, “anticipate”, “believe”, “intend”, “estimate”, “predict”, “potential”, “continue” or other similar expressions concerning matters that are not statements about the present or historical facts. Forward-looking statements in this presentation include, among other things, or statements about: our ability to conduct all required clinical and nonclinical trials for CaPre, including the timing and results of those clinical trials; whether our recently announced non-binding term sheet with a Chinese pharmaceutical company to commercialize CaPre in certain Asian countries will lead to a definitive agreement and any related payments to us; our strategy, future operations, prospects and the plans of our management; the design, regulatory plan, timeline, costs and results of our clinical and nonclinical trials for CaPre; the timing and outcome of our meetings and discussions with the U.S. Food and Drug Administration, or FDA; our planned regulatory filings for CaPre, and their timing; our expectation that our Bridging Study (as defined below) results will support our plan to get authorization from the FDA to use its 505(b)(2) pathway with new chemical entity, or NCE, status towards a New Drug Application, or NDA, approval in the United States; the timing and results from two competitor outcomes studies in high hypertriglyceridemia, or HTG, patients; the potential benefits and risks of CaPre as compared to other products in the pharmaceutical, medical food and natural health products markets; our anticipated marketing advantages and product differentiation of CaPre and its potential to become a best-in-class omega-3, or OM3, compound for the treatment of severe HTG; our estimates of the size of the potential market for CaPre, unmet medical needs in that market, the potential for market expansion, and the rate and degree of market acceptance of CaPre if it reaches commercialization, and our ability to serve that market; the potential to expand CaPre’s indication for the treatment of high TGs; the degree to which physicians would switch their patients to a product with CaPre’s target product profile; our strategy and ability to develop, commercialize and distribute CaPre in the United States and elsewhere; the manufacturing scale-up of CaPre and the related timing; the availability, consistency and sources of our raw materials, including krill oil; our expectation to be able to rely on third parties to manufacture CaPre whose manufacturing processes and facilities are in compliance with current good manufacturing practices, or cGMP; the potential for OM3s in other cardiovascular medicine, or CVM, indications; and our intention to pursue development and/or distribution partnerships to support the development and commercialization of CaPre, and to pursue strategic opportunities to provide capital and market access. All forward-looking statements reflect on our beliefs and assumptions based on information available at the time the assumption was made. All of the forward-looking statements in this presentation are qualified by this cautionary statement. There can be no guarantee that the results or developments that we anticipate will be realized or, even if substantially realized, that they will have the consequences or effects on our business, financial condition or results of operations that we anticipate. As a result, you should not place undue reliance on the forward-looking statements. Except as required by applicable law, we do not undertake to update or amend any forward-looking statements, whether as a result of new information, future events or otherwise. All forward-looking statements are made as of the date of this presentation. You should read the “Risk Factors” section of the prospectus we have filed for this offering for an understanding of the various factors that could negatively affect the accuracy of our forward-looking statements made in this presentation. The information in this presentation concerning Acasti is qualified in its entirety by the information contained in the registration statement we have filed in connection with this offering. Acasti has filed a registration statement (including a prospectus) with the U.S. Securities and Exchange Commission (SEC) for the offering to which this presentation relates. Before you invest, you should read the prospectus in that registration statement and other documents that Acasti has filed with the SEC for more complete information about Acasti and this offering. You may get these documents for free by visiting EDGAR on the SEC’s website at www.sec.gov. Alternatively, any underwriter or dealer participating in the offering will arrange to send you the prospectus if you request it by calling The Benchmark Company, LLC at 415-341-3335 or Dawson James Securities, Inc. at 743-223-8526. No prospectus has been filed in Canada and accordingly no securities of Acasti may be offered or sold in Canada in connection with the offering, except in reliance on a private placement exemption, as may be set forth in a Canadian offering memorandum supplementing the disclosure of the registration statement and prospectus referenced above. COMPANY OVERVIEW

Overview of Acasti Pharma 3 Biopharmaceutical company: spun out of Neptune Technologies in 2008 to develop innovative drugs for cardiometabolic diseases Headquartered in Montreal, Quebec, Canada Listed as ACST on TSXV and NASDAQ: first public offering late 2013 Phase 3-ready lead drug candidate, CaPre® is a uniquely formulated omega-3 (OM3) phospholipid ester being developed to treat Hypertriglyceridemia (HTG): Patented formulation cannot be synthesized or easily copied New Leadership Team in 2016: Board and expanded management team have demonstrated success in global drug development and commercialization, strategic partnering and M&A COMPANY OVERVIEW
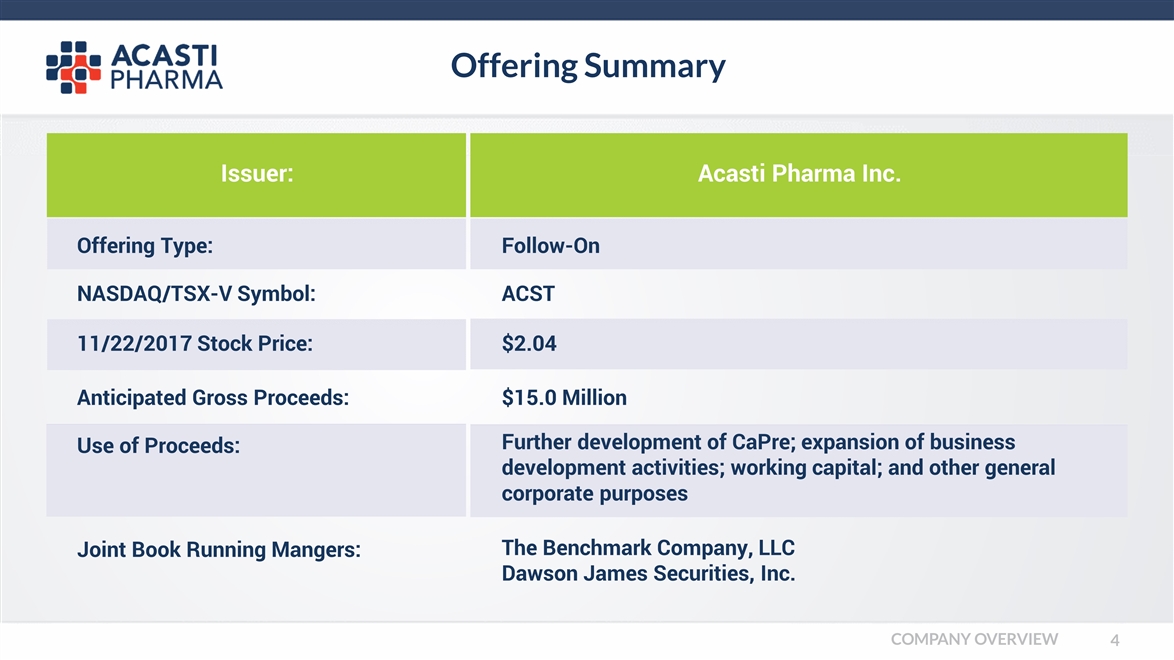
Offering Summary Offering Type: Follow-On NASDAQ/TSX-V Symbol: ACST 11/22/2017 Stock Price: Anticipated Gross Proceeds: $15.0 Million Use of Proceeds: Further development of CaPre; expansion of business development activities; working capital; and other general corporate purposes Joint Book Running Mangers: The Benchmark Company, LLC Dawson James Securities, Inc. Issuer: Acasti Pharma Inc. $2.04 COMPANY OVERVIEW

Key Investor Considerations $2.3 Billion Global Prescription OM3 Market, $750M in the US (2015) Five million scripts written annually, only two branded competitors with ~ 30% price differential with generics CaPre’s initial indication targets 3 - 4M patients with severe HTG in the US Underserved Market with Significant Expansion Opportunity HTG affects about one-third of the U.S. population, however less than 4% currently receive treatment Opportunity to expand to 40M US patients with TG >200 with positive REDUCE-IT or STRENGTH outcomes trials Late Stage Drug Assets are Rare and Valuable Acasti is currently developing opportunities for multiple commercialization partnerships Initial focus on major pharmaceutical companies in Ex-US markets; US focus post REDUCE-IT results Global IP Portfolio Has Created Strong Barriers to Competition: Novel Formulation Patented phospholipid ester/free fatty acid formulation is key to “trifecta” clinical profile and no “food effect” 20 issued patents and 15 pending applications, with protection until 2031 Plus proprietary manufacturing processes, methods, trade-secrets and know-how CaPre is Phase 3 Ready Four clinical trials involving 773 patients demonstrated positive results Potential “best-in-class” drug, targets complete “trifecta” lipid management solution, plus no “food effect” 505(b)(2) pathway speeds development by leveraging extensive safety database of LOVAZA COMPANY OVERVIEW

Recent Developments Execution of non-binding term sheet with a leading Chinese pharmaceutical partner: Term Sheet contemplates payments to Acasti of: Completion of transaction subject to further negotiation and execution of definitive agreement US$8.0 million upon signing Potential additional regulatory and commercial milestone payments in excess of US$125 million Tiered double-digit royalties on net-sales Confirmation received from FDA for Phase 3 CMC and Clinical plans Two cGMP production lots of CaPre successfully manufactured Phase 3 program initiated, on schedule for site activation by year-end CRO selected, and renowned Tufts cardiologist, Dr. Mozaffarian announced as our Principal Investigator Term to be later of (i) 5th anniversary of the last to expire Patent, or (ii) 2035, renewable for one term of 10 years COMPANY OVERVIEW
Hypertriglyceridemia: Levels elevated >150 mg/dL 40M people in US have TG levels >200mg/dL and should be treated ~4M people in US w/TG levels >500mg/dl (CaPre’s first indication) at greater risk of diabetes, heart disease, renal failure, and pancreatitis Hypertriglyceridemia is a Large and Undertreated Problem Triglycerides (TGs) Type of fat found in the blood; in excess stored in fat cells Genetics, high fat diets, diabetes, obesity, smoking, and sedentary habits can cause high TG levels Clinical, epidemiological and genetic studies suggest high levels of TGs increase Coronary Artery Disease risk at essentially the same rate as bad cholesterol (LDL-C) Statins for high LDL-C, together with OM3s and/or fibrates for HTG, are now the standard of care: Fibrates and niacin have been used to treat HTG, but both cause serious side effects While statins are widely used, <4% of HTG patients are prescribed a fibrate or OM3 COMPANY OVERVIEW
CaPre’s Novel Formulation Combines Phospholipids and Free Fatty Acid Forms of EPA and DHA A novel formulation of polyunsaturated fatty acids, primarily OM3s (principally EPA and DHA), delivered as phospholipid esters and free fatty acids for rapid absorption Essential components are extracted and purified from krill, which are naturally rich in OM3 phospholipids Krill → Raw Krill oil (RKO) → NKPL66 (Drug Substance/API) → CaPre (Drug Product) CaPre is supplied as a 1-gram hard-gel capsule for oral administration Each 1 gram capsule of CaPre® contains approximately 310 mg of EPA and DHA (expressed as free fatty acids) First indication: for the treatment of severe hypertriglyceridemia COMPANY OVERVIEW
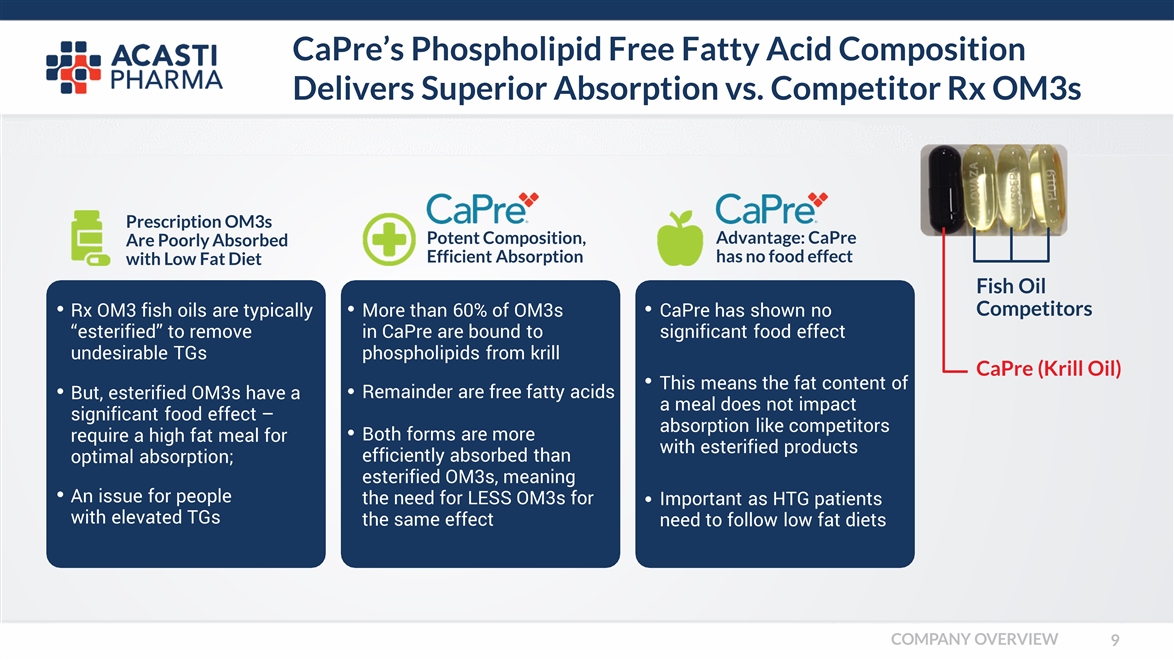
CaPre’s Phospholipid Free Fatty Acid Composition Delivers Superior Absorption vs. Competitor Rx OM3s Prescription OM3s Are Poorly Absorbed with Low Fat Diet Potent Composition, Efficient Absorption Advantage: CaPre has no food effect But, esterified OM3s have a significant food effect – require a high fat meal for optimal absorption; An issue for people with elevated TGs Rx OM3 fish oils are typically “esterified” to remove undesirable TGs Remainder are free fatty acids Both forms are more efficiently absorbed than esterified OM3s, meaning the need for LESS OM3s for the same effect More than 60% of OM3s in CaPre are bound to phospholipids from krill This means the fat content of a meal does not impact absorption like competitors with esterified products Important as HTG patients need to follow low fat diets CaPre has shown no significant food effect CaPre (Krill Oil) Fish Oil Competitors COMPANY OVERVIEW
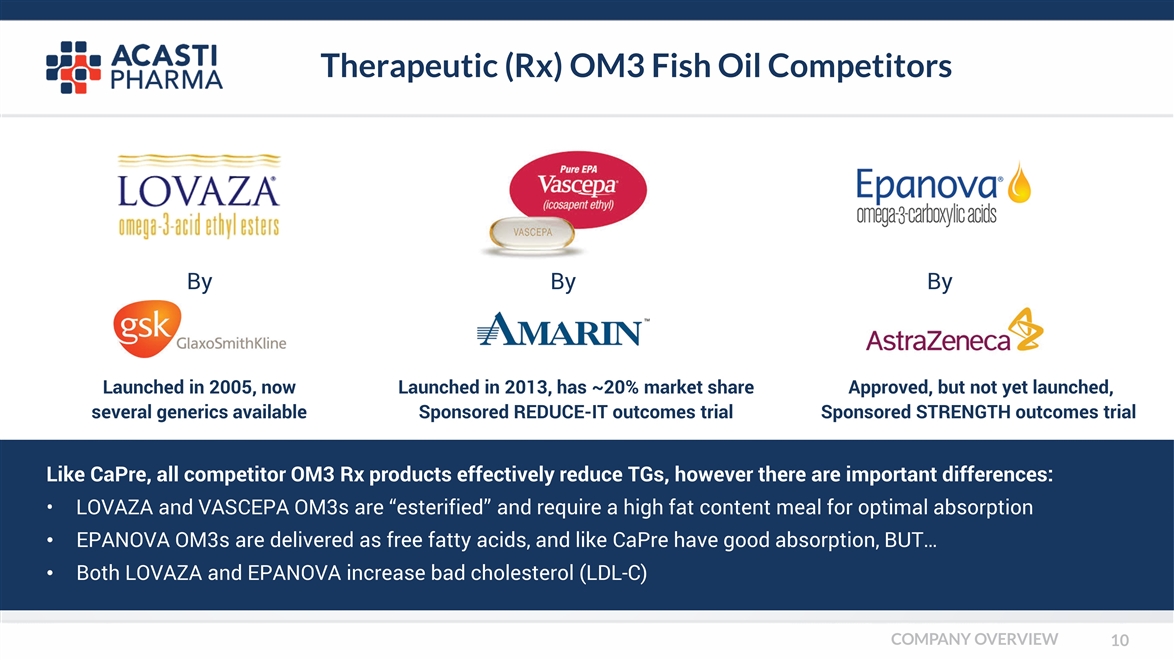
Therapeutic (Rx) OM3 Fish Oil Competitors Like CaPre, all competitor OM3 Rx products effectively reduce TGs, however there are important differences: • LOVAZA and VASCEPA OM3s are “esterified” and require a high fat content meal for optimal absorption EPANOVA OM3s are delivered as free fatty acids, and like CaPre have good absorption, BUT… Both LOVAZA and EPANOVA increase bad cholesterol (LDL-C) By By By Launched in 2005, now several generics available Launched in 2013, has ~20% market share Sponsored REDUCE-IT outcomes trial Approved, but not yet launched, Sponsored STRENGTH outcomes trial COMPANY OVERVIEW
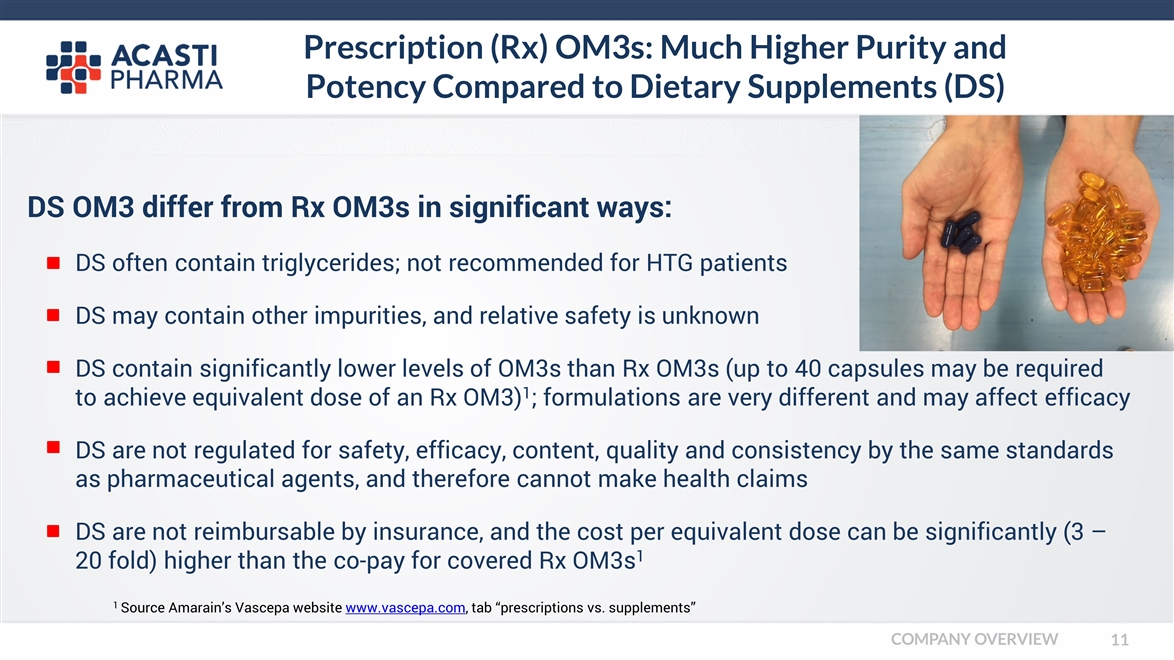
Prescription (Rx) OM3s: Much Higher Purity and Potency Compared to Dietary Supplements (DS) DS OM3 differ from Rx OM3s in significant ways: DS often contain triglycerides; not recommended for HTG patients DS may contain other impurities, and relative safety is unknown DS contain significantly lower levels of OM3s than Rx OM3s (up to 40 capsules may be required to achieve equivalent dose of an Rx OM3)1; formulations are very different and may affect efficacy DS are not regulated for safety, efficacy, content, quality and consistency by the same standards as pharmaceutical agents, and therefore cannot make health claims DS are not reimbursable by insurance, and the cost per equivalent dose can be significantly (3 – 20 fold) higher than the co-pay for covered Rx OM3s1 1 Source Amarain’s Vascepa website www.vascepa.com, tab “prescriptions vs. supplements” COMPANY OVERVIEW
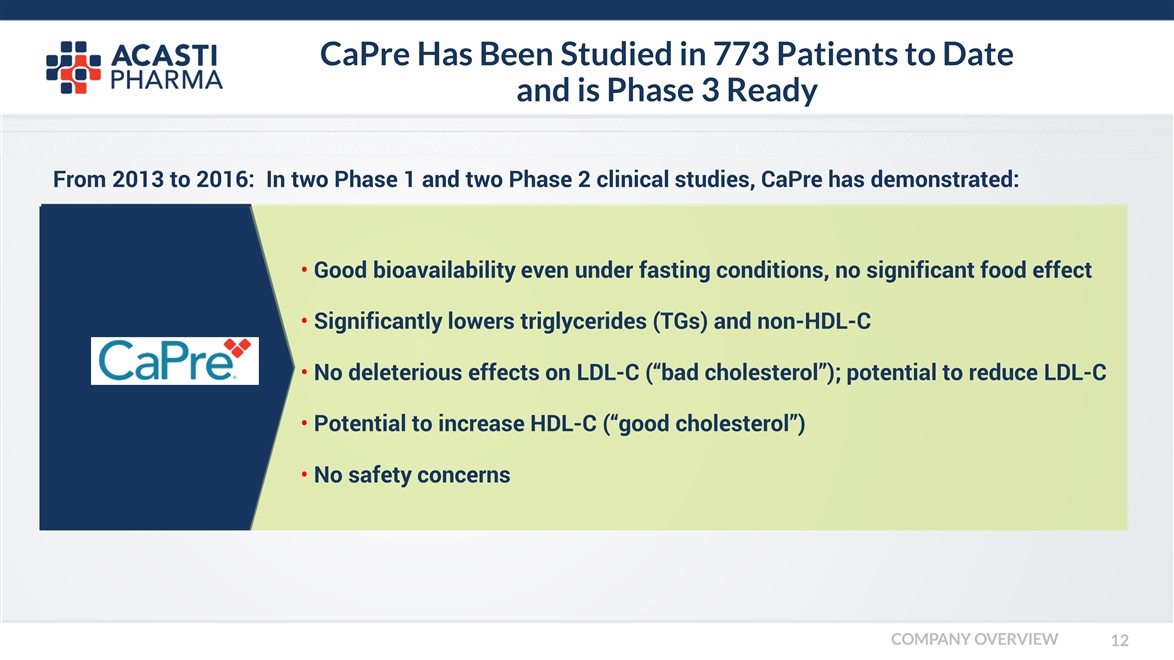
CaPre Has Been Studied in 773 Patients to Date and is Phase 3 Ready Good bioavailability even under fasting conditions, no significant food effect Significantly lowers triglycerides (TGs) and non-HDL-C No deleterious effects on LDL-C (“bad cholesterol”); potential to reduce LDL-C Potential to increase HDL-C (“good cholesterol”) No safety concerns From 2013 to 2016: In two Phase 1 and two Phase 2 clinical studies, CaPre has demonstrated: COMPANY OVERVIEW
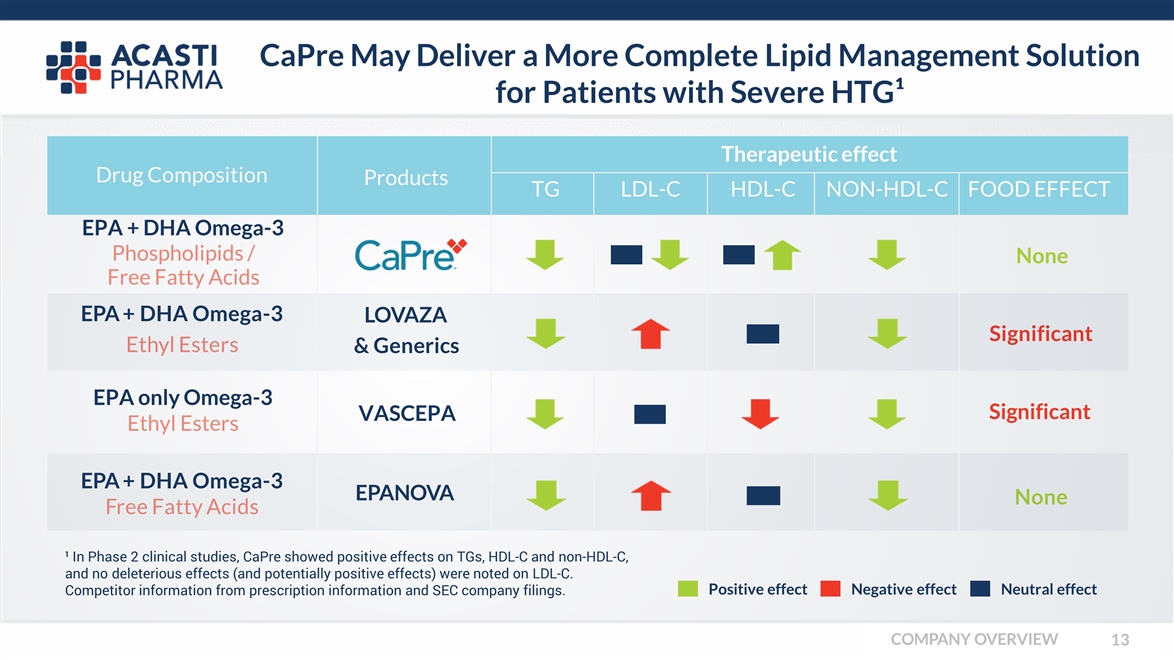
CaPre May Deliver a More Complete Lipid Management Solution for Patients with Severe HTG¹ EPA + DHA Omega-3LOVAZA Ethyl Esters & Generics Significant EPA + DHA Omega-3EPANOVA Free Fatty Acids None ¹ In Phase 2 clinical studies, CaPre showed positive effects on TGs, HDL-C and non-HDL-C, and no deleterious effects (and potentially positive effects) were noted on LDL-C. Competitor information from prescription information and SEC company filings. Drug Composition Therapeutic effect ProductsTGLDL-CHDL-CNON-HDL-CFOOD EFFECT EPA + DHA Omega-3 Phospholipids / Free Fatty Acids None EPA only Omega-3 Ethyl Esters VASCEPA Significant Positive effect Neutral effect Negative effect COMPANY OVERVIEW
Phase 3 Program: Expected to Reduce Time, Cost and Risk Clinical program reviewed and clarified with FDA; site initiation expected by end of 2017 Long term (1 year) safety data not required – PK safety bridge successfully established with LOVAZA, supporting 505(b)(2) pathway Two Phase 3 clinical studies – 6 months duration, run in parallel Multi-center, placebo controlled, randomized, double-blind design ~250 patients per trial, total ~500 patients Primary endpoint: determine efficacy of CaPre 4g/day in lowering TGs in severe HTG patients with levels between 500 – 1500 mg/dl, and confirm safety Numerous secondary and exploratory endpoints: designed to assess effect of CaPre on the lipid profile and certain metabolic, inflammatory and cardiovascular risk markers (basis for possible expanded claims and future indications) COMPANY OVERVIEW
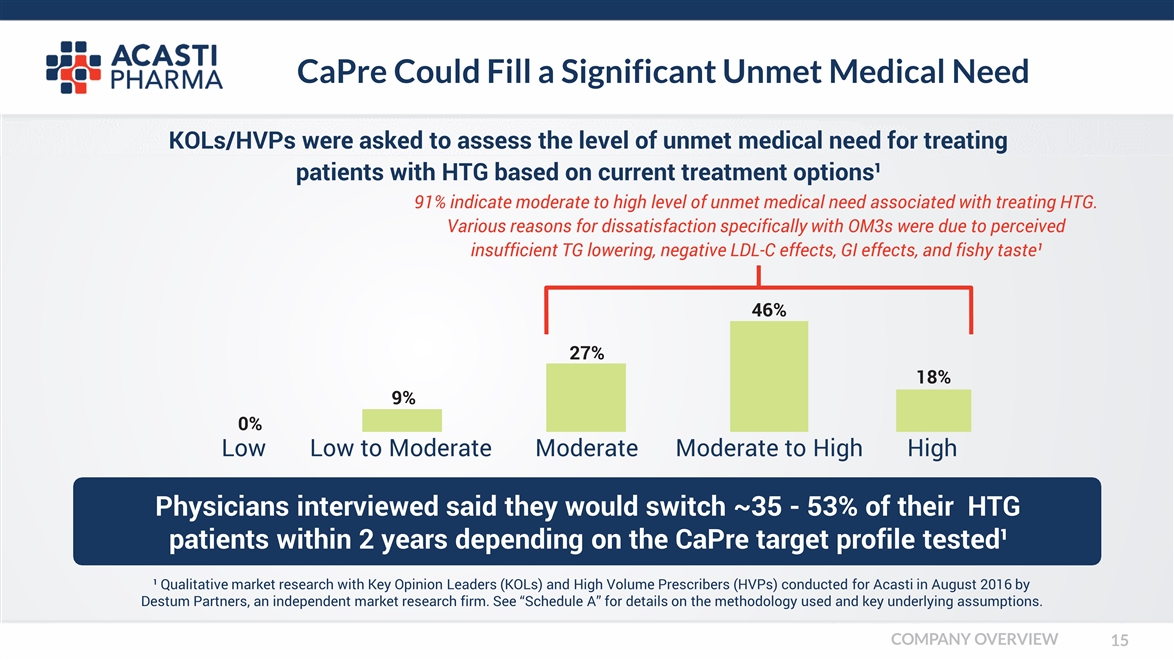
CaPre Could Fill a Significant Unmet Medical Need Physicians interviewed said they would switch ~35 - 53% of their HTG patients within 2 years depending on the CaPre target profile tested¹ Low KOLs/HVPs were asked to assess the level of unmet medical need for treating patients with HTG based on current treatment options¹ 91% indicate moderate to high level of unmet medical need associated with treating HTG. Various reasons for dissatisfaction specifically with OM3s were due to perceived insufficient TG lowering, negative LDL-C effects, GI effects, and fishy taste¹ 46% 18% 27% Low to Moderate Moderate Moderate to High High 9% 0% ¹ Qualitative market research with Key Opinion Leaders (KOLs) and High Volume Prescribers (HVPs) conducted for Acasti in August 2016 by Destum Partners, an independent market research firm. See “Schedule A” for details on the methodology used and key underlying assumptions. COMPANY OVERVIEW
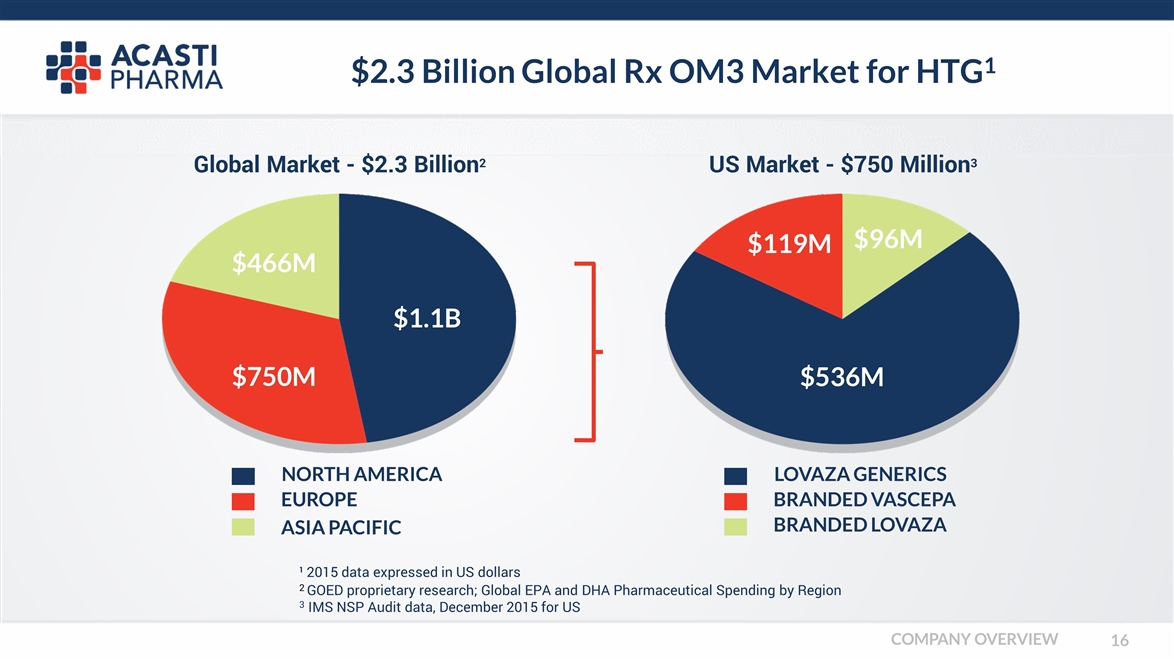
$2.3 Billion Global Rx OM3 Market for HTG1 LOVAZA GENERICS BRANDED VASCEPA BRANDED LOVAZA US Market - $750 Million3 $536M Global Market - $2.3 Billion2 $1.1B $750M $466M NORTH AMERICA EUROPE ASIA PACIFIC ¹ 2015 data expressed in US dollars 2 GOED proprietary research; Global EPA and DHA Pharmaceutical Spending by Region 3 IMS NSP Audit data, December 2015 for US $119M $96M COMPANY OVERVIEW

Attractive US Rx OM3 Market for Treating HTG Large, underserved market; HTG affects ~one-third of the U.S. population1 Only 3.6% of patients with TG ≥ 200 mg/dl are treated with TG lowering meds1,2 ~5 million scripts written each year2 Only two marketed branded competitors (LOVAZA & VASCEPA) LOVAZA generics available2, EPANOVA approved but not launched In spite of generics, in 2016 >50% of scripts were still written for branded products4 Attractive pricing and reimbursement4 While some generic switching occurs at pharmacy, only ~25% decline in total market value realized by 2015 since first generic launched in 20133 Pharmacy Benefit Managers offer “Preferred Brand” status (Tier 2 or Tier 3), without significant restrictions (i.e. no prior authorization, step edits, or high co-payments) Pricing of generics average US$190/mo., branded products US$250 - $300/mo. 1 Ford, Archives of Internal Medicine, 2009; 169(6):572-578 | 2 Kapoor and Miller, ACC, 2016 3 IMS NSP Audit data, December 2015 for US | 4 Qualitative market research conducted for Acasti in August 2016 by Destum Partners, an independent market research firm. See “Schedule A” for details on the methodology used and key underlying assumptions. COMPANY OVERVIEW
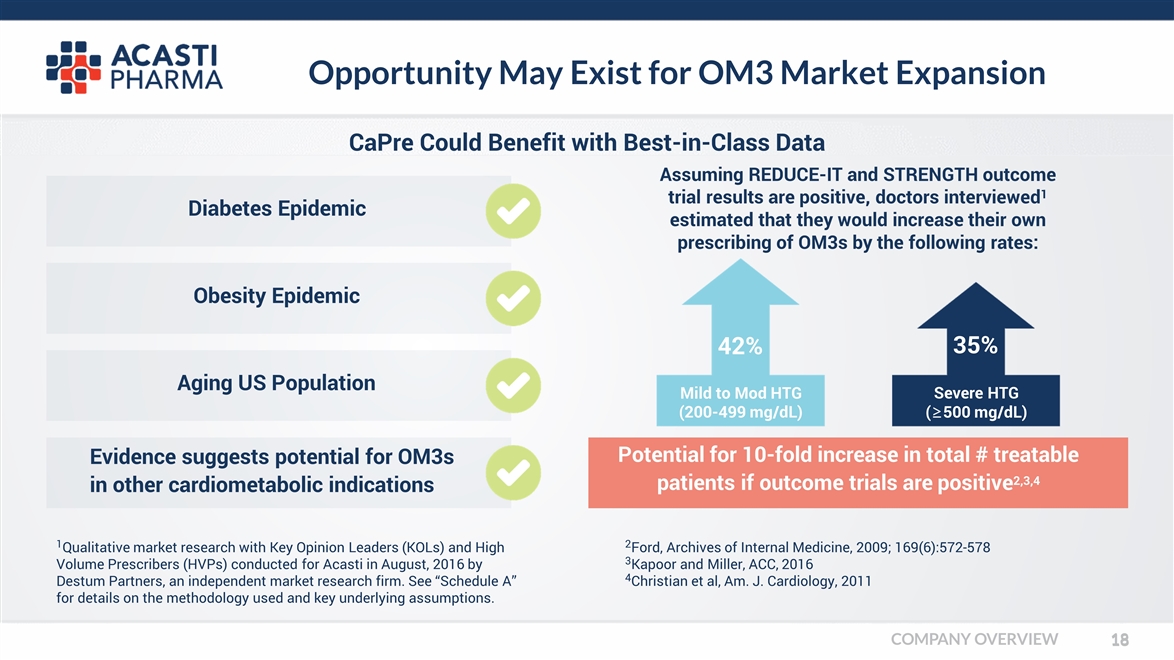
Opportunity May Exist for OM3 Market Expansion Diabetes Epidemic Obesity Epidemic Aging US Population Evidence suggests potential for OM3s in other cardiometabolic indications 1Qualitative market research with Key Opinion Leaders (KOLs) and High Volume Prescribers (HVPs) conducted for Acasti in August, 2016 by Destum Partners, an independent market research firm. See “Schedule A” for details on the methodology used and key underlying assumptions. Potential for 10-fold increase in total # treatable patients if outcome trials are positive2,3,4 35% Severe HTG (≥500 mg/dL) 42% Mild to Mod HTG (200-499 mg/dL) CaPre Could Benefit with Best-in-Class Data 2Ford, Archives of Internal Medicine, 2009; 169(6):572-578 3Kapoor and Miller, ACC, 2016 4Christian et al, Am. J. Cardiology, 2011 Assuming REDUCE-IT and STRENGTH outcome trial results are positive, doctors interviewed1 estimated that they would increase their own prescribing of OM3s by the following rates: COMPANY OVERVIEW
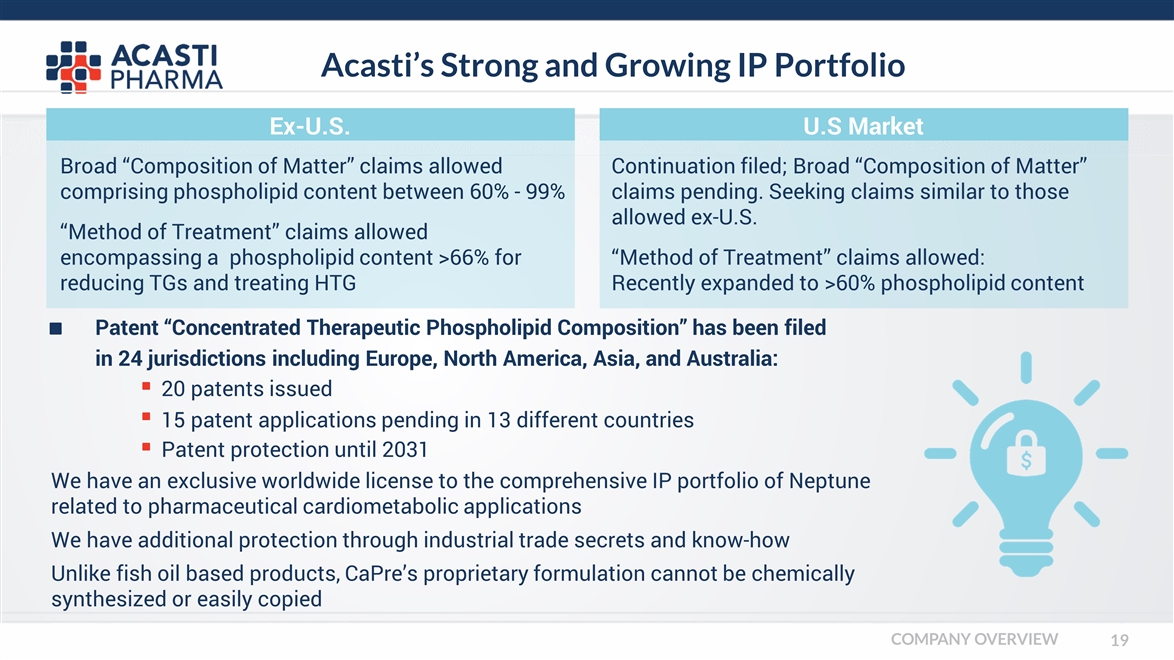
Acasti’s Strong and Growing IP Portfolio Patent “Concentrated Therapeutic Phospholipid Composition” has been filed in 24 jurisdictions including Europe, North America, Asia, and Australia: Broad “Composition of Matter” claims allowed comprising phospholipid content between 60% - 99% “Method of Treatment” claims allowed encompassing a phospholipid content >66% for reducing TGs and treating HTG Continuation filed; Broad “Composition of Matter” claims pending. Seeking claims similar to those allowed ex-U.S. “Method of Treatment” claims allowed: Recently expanded to >60% phospholipid content Ex-U.S. U.S Market 20 patents issued 15 patent applications pending in 13 different countries Patent protection until 2031 We have an exclusive worldwide license to the comprehensive IP portfolio of Neptune related to pharmaceutical cardiometabolic applications We have additional protection through industrial trade secrets and know-how Unlike fish oil based products, CaPre’s proprietary formulation cannot be chemically synthesized or easily copied COMPANY OVERVIEW
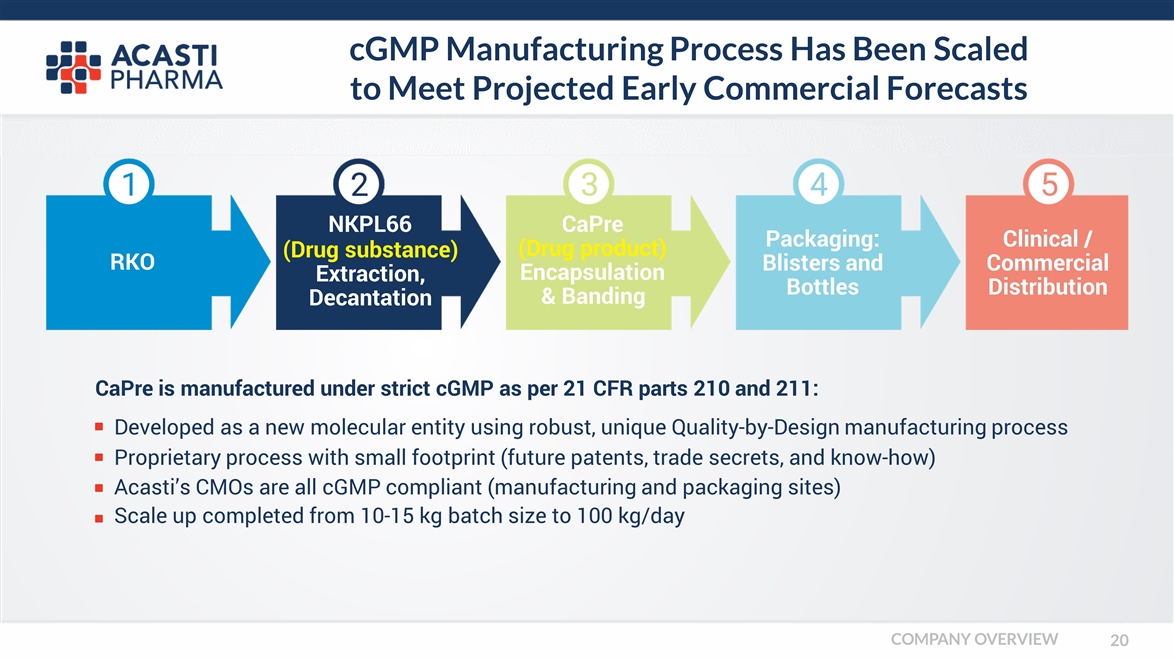
cGMP Manufacturing Process Has Been Scaled to Meet Projected Early Commercial Forecasts CaPre is manufactured under strict cGMP as per 21 CFR parts 210 and 211: RKO NKPL66 (Drug substance) Extraction, Decantation CaPre (Drug product) Encapsulation & Banding Packaging: Blisters and Bottles Clinical / Commercial Distribution Developed as a new molecular entity using robust, unique Quality-by-Design manufacturing process Proprietary process with small footprint (future patents, trade secrets, and know-how) Acasti’s CMOs are all cGMP compliant (manufacturing and packaging sites) Scale up completed from 10-15 kg batch size to 100 kg/day COMPANY OVERVIEW
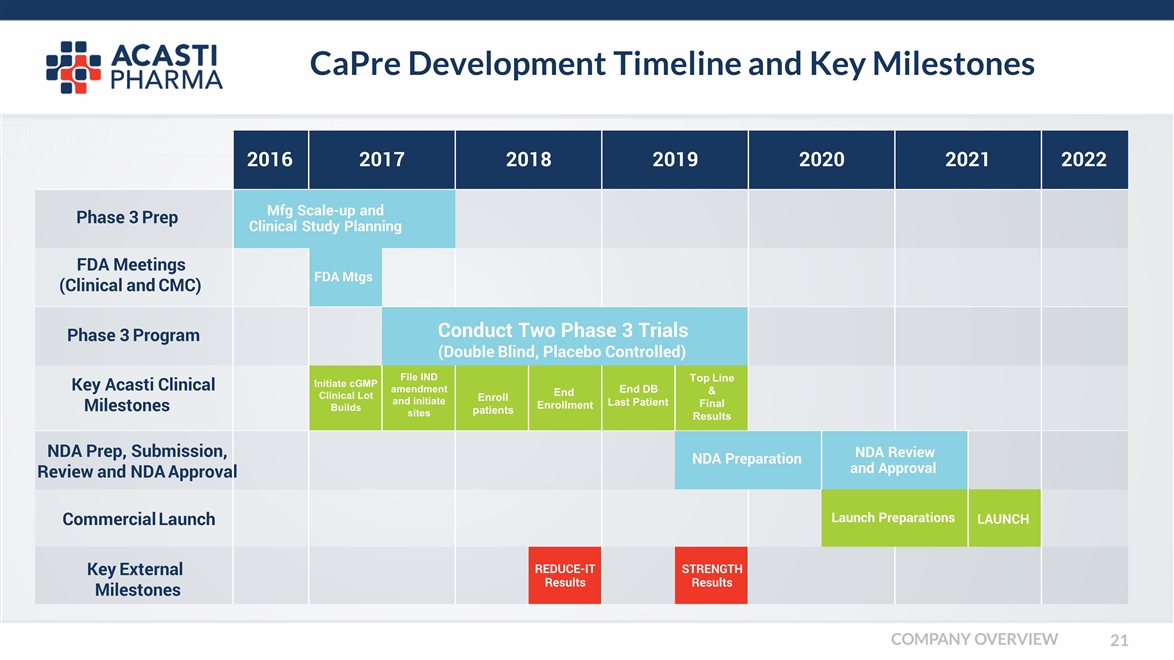
CaPre Development Timeline and Key Milestones 2016 2017 2018 2019 2020 2021 2022 Mfg Scale-up and Clinical Study Planning FDA Mtgs Conduct Two Phase 3 Trials (Double Blind, Placebo Controlled) Initiate cGMP Clinical Lot Builds File IND amendment and initiate sites Enroll patients End Enrollment End DB Last Patient Top Line & Final Results NDA Preparation NDA Review and Approval Launch Preparations LAUNCH REDUCE-IT Results STRENGTH Results Phase 3 Prep FDA Meetings (Clinical and CMC) Phase 3 Program Key Acasti Clinical Milestones NDA Prep, Submission, Review and NDA Approval Commercial Launch Key External Milestones COMPANY OVERVIEW

Strengthened Acasti Leadership Team Board of Directors Rick Schottenfeld Director Dr. Roderick Carter, MD Chairman of Board Senior Management John Canan Audit Committee Chair Jan D’Alvise President & CEO, Acasti Katherine Crewe Director Jan D’Alvise President & CEO Linda O’Keefe, CPA CFO Pierre Lemieux, PhD COO Laurent Harvey, B.Pharm., M.Sc. VP Clinical and Non-clinical Affairs COMPANY OVERVIEW
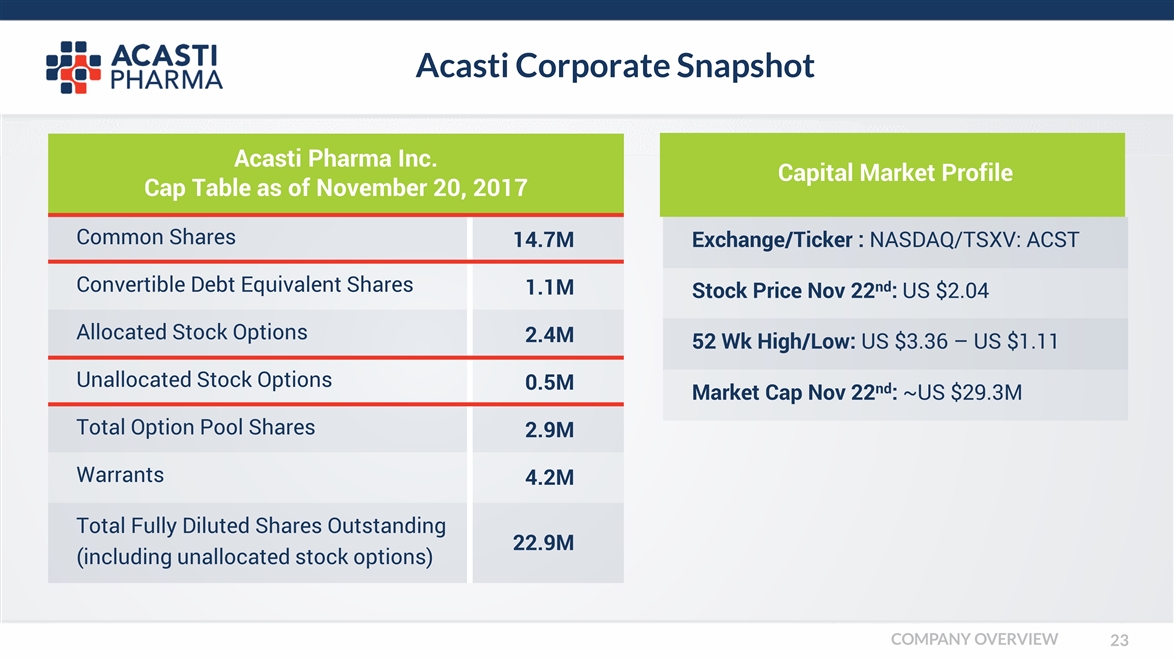
Acasti Pharma Inc. Cap Table as of November 20, 2017 Acasti Corporate Snapshot Common Shares Exchange/Ticker : NASDAQ/TSXV: ACST 14.7M Convertible Debt Equivalent Shares Stock Price Nov 22nd: US $2.04 1.1M Allocated Stock Options 52 Wk High/Low: US $3.36 – US $1.11 2.4M Unallocated Stock Options Market Cap Nov 22nd: ~US $29.3M 0.5M Total Option Pool Shares 2.9M Warrants 4.2M Total Fully Diluted Shares Outstanding (including unallocated stock options) 22.9M Capital Market Profile COMPANY OVERVIEW

Why Invest in Acasti? Highly Differentiated and Proprietary Asset Targets “Trifecta” effect on blood lipids, and no food effect Strong and growing IP portfolio Developing global partnering opportunities Well Positioned Experienced and Accomplished Management, Board of Directors and Scientific Advisors Winning Team Promising Phase 1 & 2 Clinical Results, Phase 3 Ready CaPre; potential "Best-in-Class" Omega-3 therapeutic Phase 3 Ready $2.3B Current Market with Opportunity to Expand Significantly in Just a Few Years Growing Market Growing Market COMPANY OVERVIEW

Clinical Data Summaries COMPANY OVERVIEW
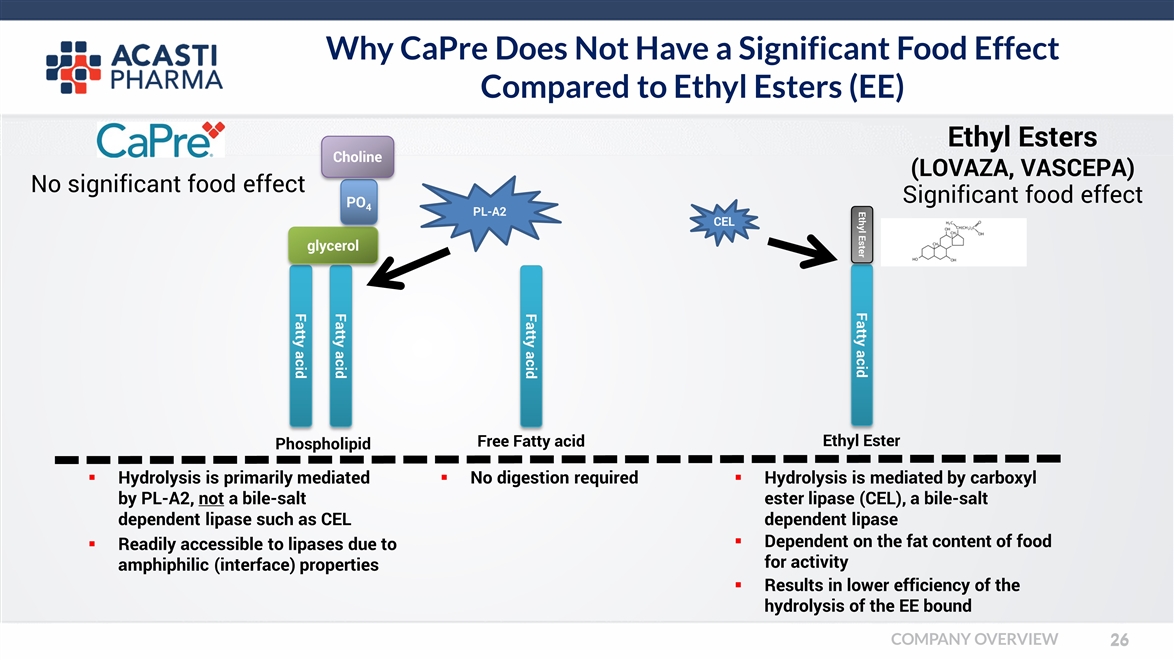
Why CaPre Does Not Have a Significant Food Effect Compared to Ethyl Esters (EE) Fatty acid Ethyl Ester Ethyl Ester Fatty acid Fatty acid glycerol PO4 Choline Fatty acid Phospholipid Free Fatty acid Ethyl Esters (LOVAZA, VASCEPA) Hydrolysis is mediated by carboxyl ester lipase (CEL), a bile-salt dependent lipase Dependent on the fat content of food for activity No digestion required Hydrolysis is primarily mediated by PL-A2, not a bile-salt dependent lipase such as CEL PL-A2 CEL Readily accessible to lipases due to amphiphilic (interface) properties Results in lower efficiency of the hydrolysis of the EE bound No significant food effect Significant food effect COMPANY OVERVIEW
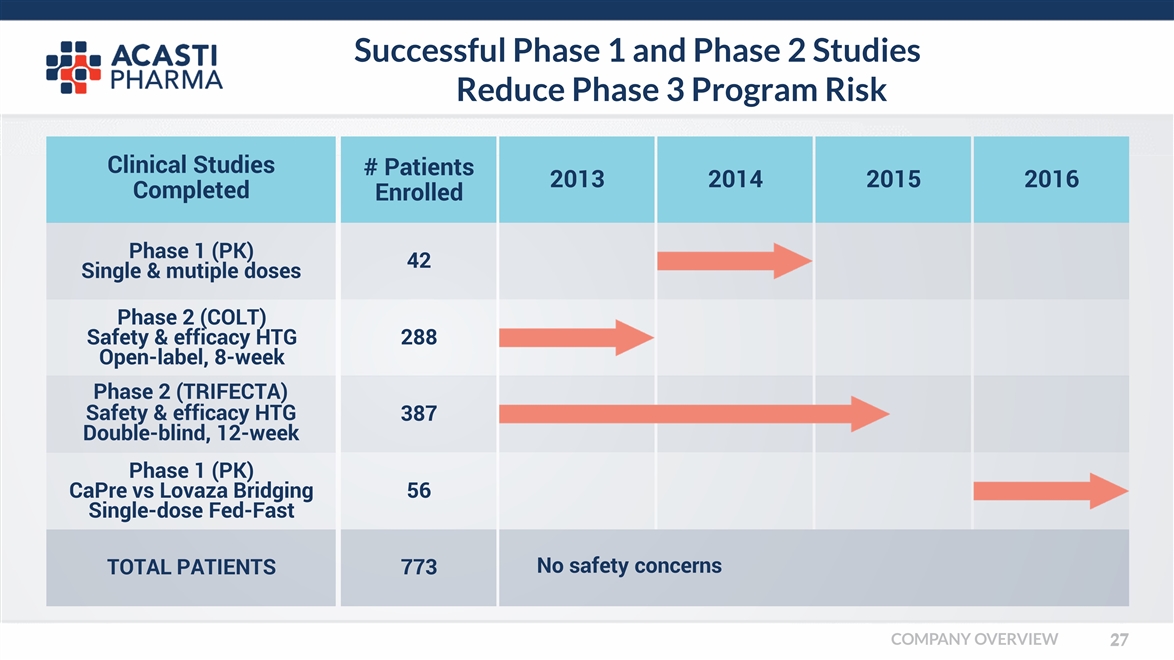
Successful Phase 1 and Phase 2 Studies Reduce Phase 3 Program Risk Clinical Studies Completed # Patients Enrolled 2013 2014 2015 2016 Phase 1 (PK) Single & mutiple doses Phase 2 (COLT) Safety & efficacy HTG Open-label, 8-week Phase 2 (TRIFECTA) Safety & efficacy HTG Double-blind, 12-week Phase 1 (PK) CaPre vs Lovaza Bridging Single-dose Fed-Fast TOTAL PATIENTS 42 288 387 56 773 No safety concerns COMPANY OVERVIEW
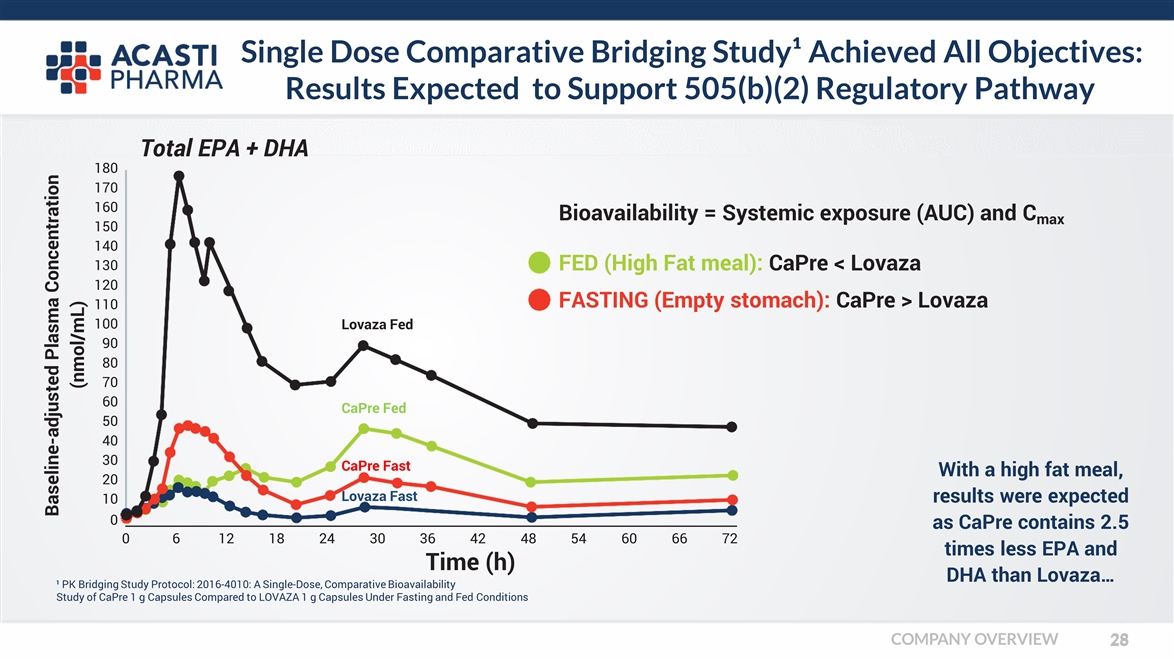
Single Dose Comparative Bridging Study¹ Achieved All Objectives: Results Expected to Support 505(b)(2) Regulatory Pathway ¹ PK Bridging Study Protocol: 2016-4010: A Single-Dose, Comparative Bioavailability Study of CaPre 1 g Capsules Compared to LOVAZA 1 g Capsules Under Fasting and Fed Conditions Bioavailability = Systemic exposure (AUC) and Cmax With a high fat meal, results were expected as CaPre contains 2.5 times less EPA and DHA than Lovaza… Baseline-adjusted Plasma Concentration (nmol/mL) Total EPA + DHA 0 180 170 160 150 140 130 120 110 100 90 80 70 60 50 40 30 20 10 0612182430 36424854606672 Time (h) FED (High Fat meal): CaPre < Lovaza FASTING (Empty stomach): CaPre > Lovaza Lovaza Fed CaPre Fed CaPre Fast Lovaza Fast COMPANY OVERVIEW
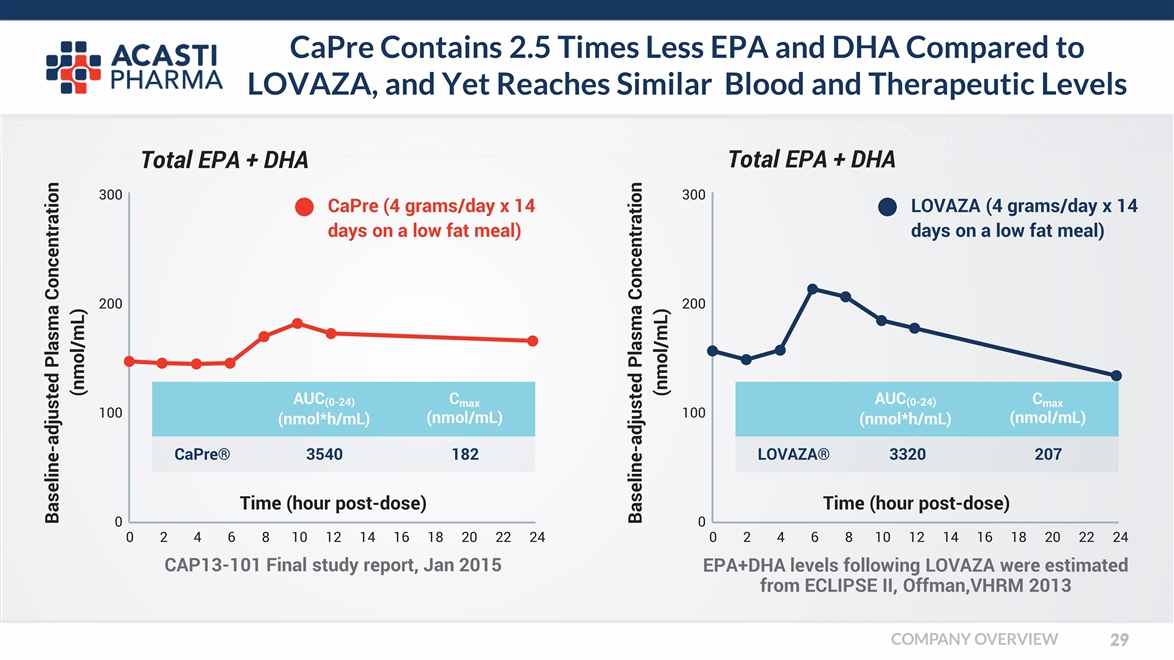
CaPre Contains 2.5 Times Less EPA and DHA Compared to LOVAZA, and Yet Reaches Similar Blood and Therapeutic Levels Baseline-adjusted Plasma Concentration (nmol/mL) 0 100 200 300 CaPre (4 grams/day x 14 days on a low fat meal) Time (hour post-dose) 024681012141618202224 CAP13-101 Final study report, Jan 2015 AUC(0-24) (nmol*h/mL) Cmax (nmol/mL) CaPre® 3540 182 Baseline-adjusted Plasma Concentration (nmol/mL) Total EPA + DHA 0 Time (hour post-dose) 024681012141618202224 100 200 300 LOVAZA (4 grams/day x 14 days on a low fat meal) EPA+DHA levels following LOVAZA were estimated from ECLIPSE II, Offman,VHRM 2013 AUC(0-24) (nmol*h/mL) Cmax (nmol/mL) LOVAZA® 3320 207 Total EPA + DHA COMPANY OVERVIEW
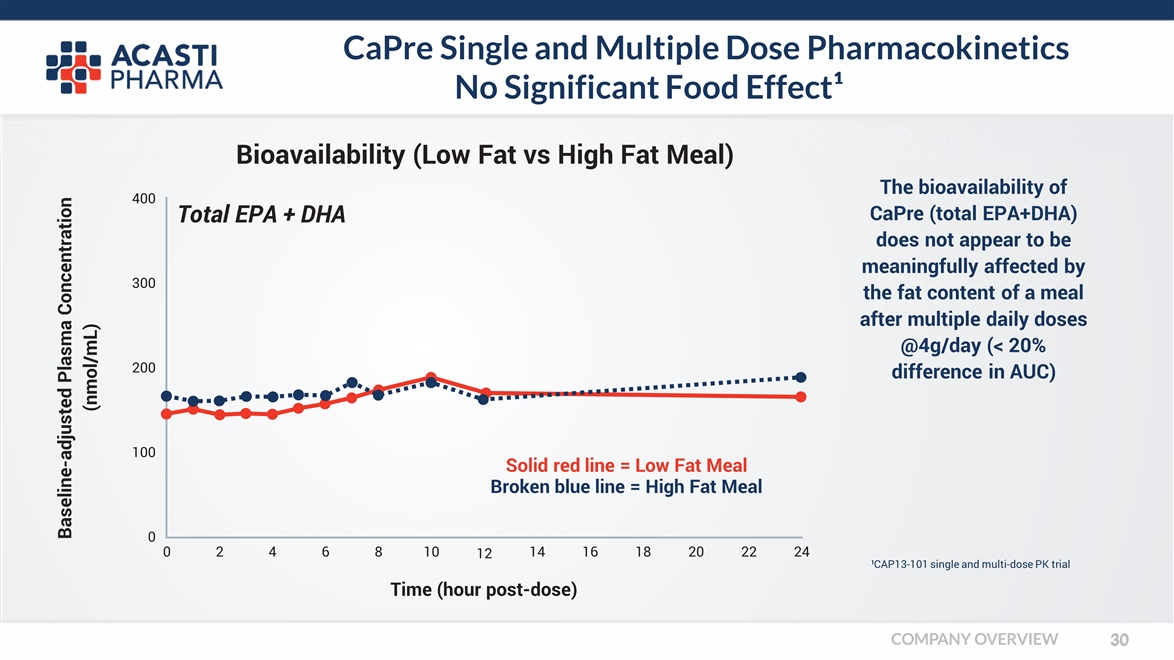
CaPre Single and Multiple Dose Pharmacokinetics No Significant Food Effect¹ The bioavailability of CaPre (total EPA+DHA) does not appear to be meaningfully affected by the fat content of a meal after multiple daily doses @4g/day (< 20% difference in AUC) Baseline-adjusted Plasma Concentration (nmol/mL) Time (hour post-dose) Bioavailability (Low Fat vs High Fat Meal) Total EPA + DHA 0 0 2 4 6 8 12 1014 16 18 20 22 24 100 200 300 400 Solid red line = Low Fat Meal Broken blue line = High Fat Meal ¹CAP13-101 single and multi-dose PK trial COMPANY OVERVIEW
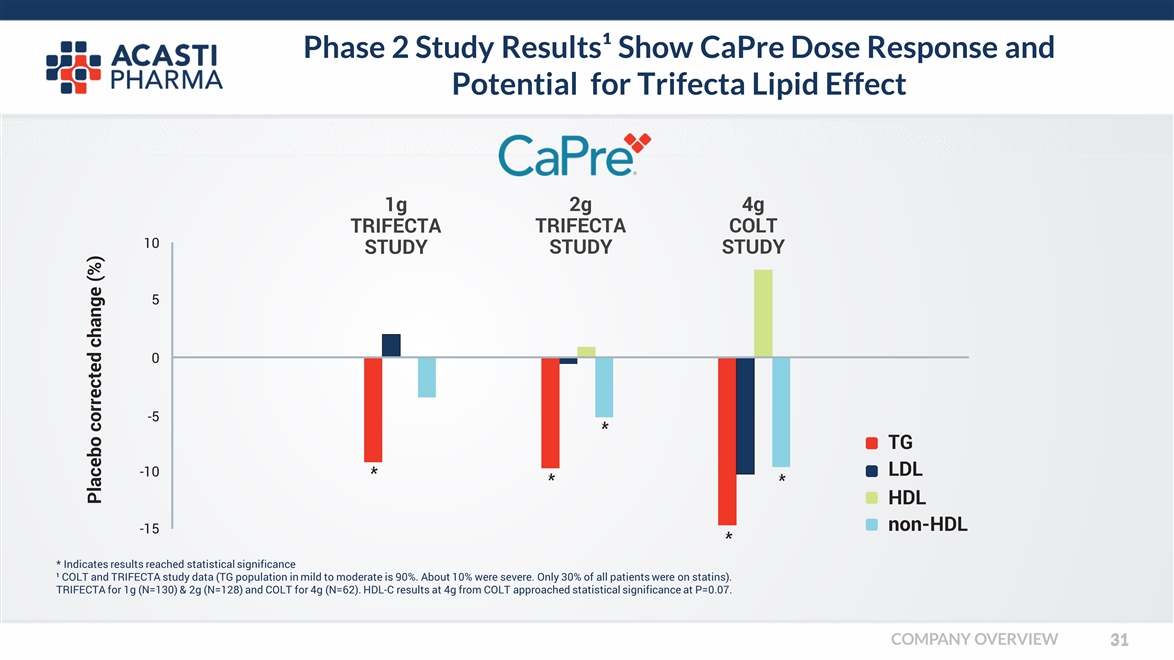
Phase 2 Study Results¹ Show CaPre Dose Response and Potential for Trifecta Lipid Effect * Indicates results reached statistical significance ¹ COLT and TRIFECTA study data (TG population in mild to moderate is 90%. About 10% were severe. Only 30% of all patients were on statins). TRIFECTA for 1g (N=130) & 2g (N=128) and COLT for 4g (N=62). HDL-C results at 4g from COLT approached statistical significance at P=0.07. Placebo corrected change (%) -15 -10 5 0 -5 10 TG LDL HDL non-HDL 1g TRIFECTA STUDY 2g TRIFECTA STUDY 4g COLT STUDY COMPANY OVERVIEW
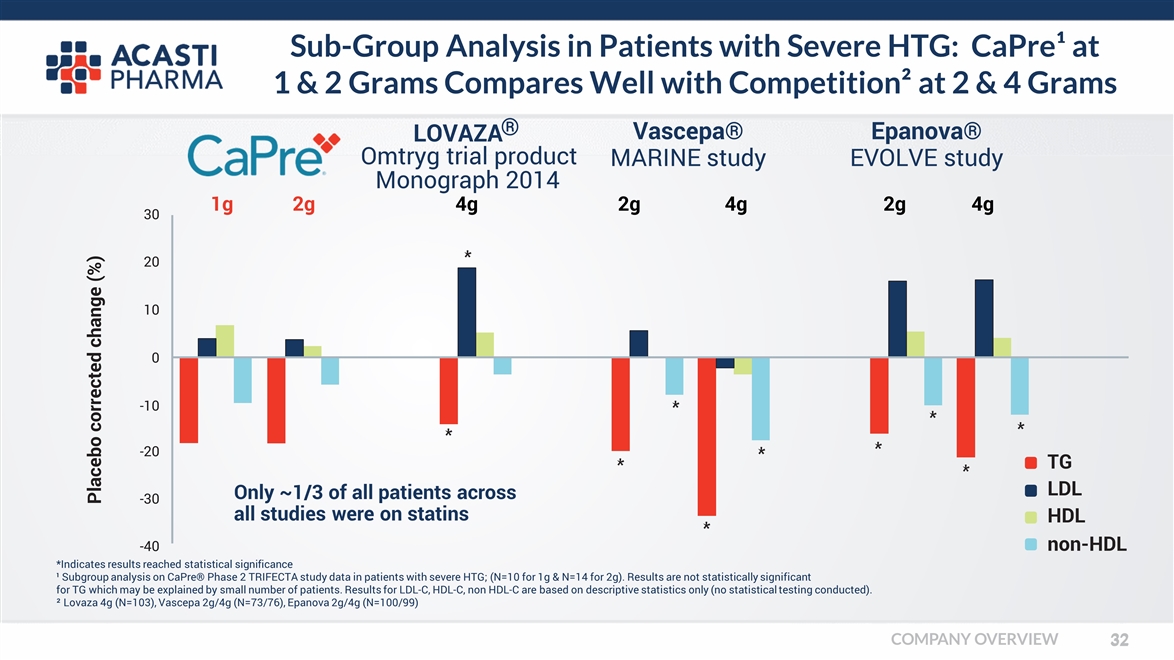
Sub-Group Analysis in Patients with Severe HTG: CaPre¹ at 1 & 2 Grams Compares Well with Competition² at 2 & 4 Grams *Indicates results reached statistical significance ¹ Subgroup analysis on CaPre® Phase 2 TRIFECTA study data in patients with severe HTG; (N=10 for 1g & N=14 for 2g). Results are not statistically significant for TG which may be explained by small number of patients. Results for LDL-C, HDL-C, non HDL-C are based on descriptive statistics only (no statistical testing conducted). ² Lovaza 4g (N=103), Vascepa 2g/4g (N=73/76), Epanova 2g/4g (N=100/99) LOVAZA Omtryg trial product 1g Monograph 2014 2g Vascepa® MARINE study Epanova® EVOLVE study Placebo corrected change (%) -40 -30 -20 20 10 0 -10 30 TG LDL HDL non-HDL Only ~1/3 of all patients across all studies were on statins 4g 2g 4g 2g 4g ® COMPANY OVERVIEW
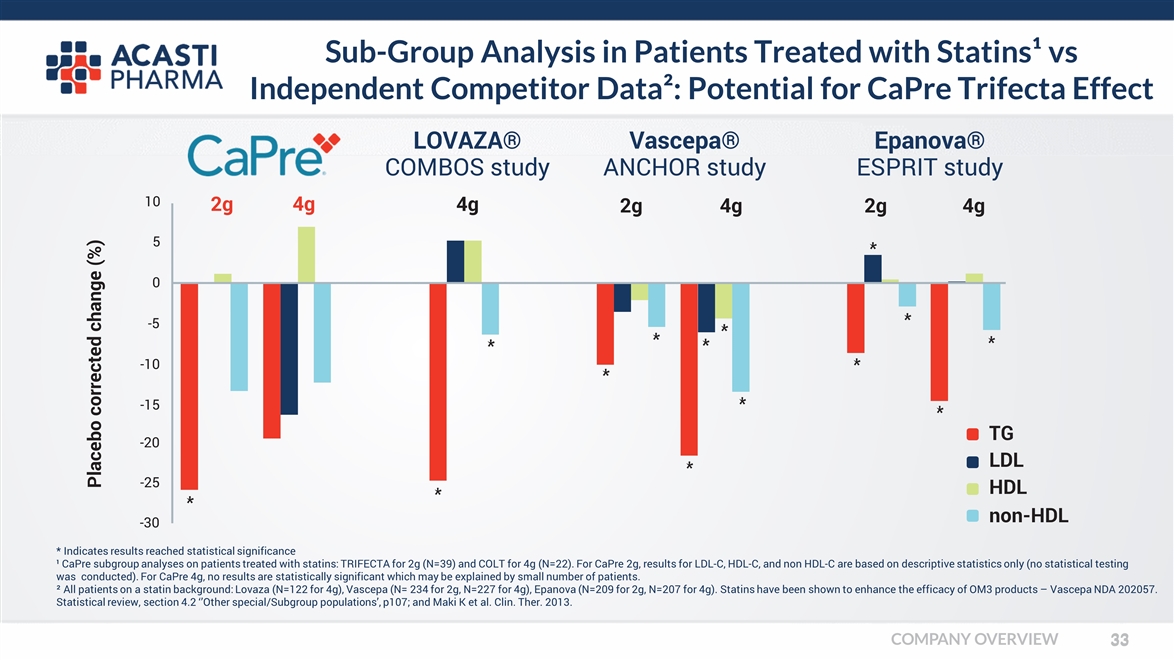
* Indicates results reached statistical significance ¹ CaPre subgroup analyses on patients treated with statins: TRIFECTA for 2g (N=39) and COLT for 4g (N=22). For CaPre 2g, results for LDL-C, HDL-C, and non HDL-C are based on descriptive statistics only (no statistical testing was conducted). For CaPre 4g, no results are statistically significant which may be explained by small number of patients. ² All patients on a statin background: Lovaza (N=122 for 4g), Vascepa (N= 234 for 2g, N=227 for 4g), Epanova (N=209 for 2g, N=207 for 4g). Statins have been shown to enhance the efficacy of OM3 products – Vascepa NDA 202057. Statistical review, section 4.2 ‘’Other special/Subgroup populations’, p107; and Maki K et al. Clin. Ther. 2013. 2g LOVAZA® COMBOS study 4g 4g Vascepa® ANCHOR study 2g4g Epanova® ESPRIT study 2g4g Placebo corrected change (%) -25 -20 5 0 -5 -10 -15 10 -30 TG LDL HDL non-HDL Sub-Group Analysis in Patients Treated with Statins¹ vs Independent Competitor Data²: Potential for CaPre Trifecta Effect COMPANY OVERVIEW
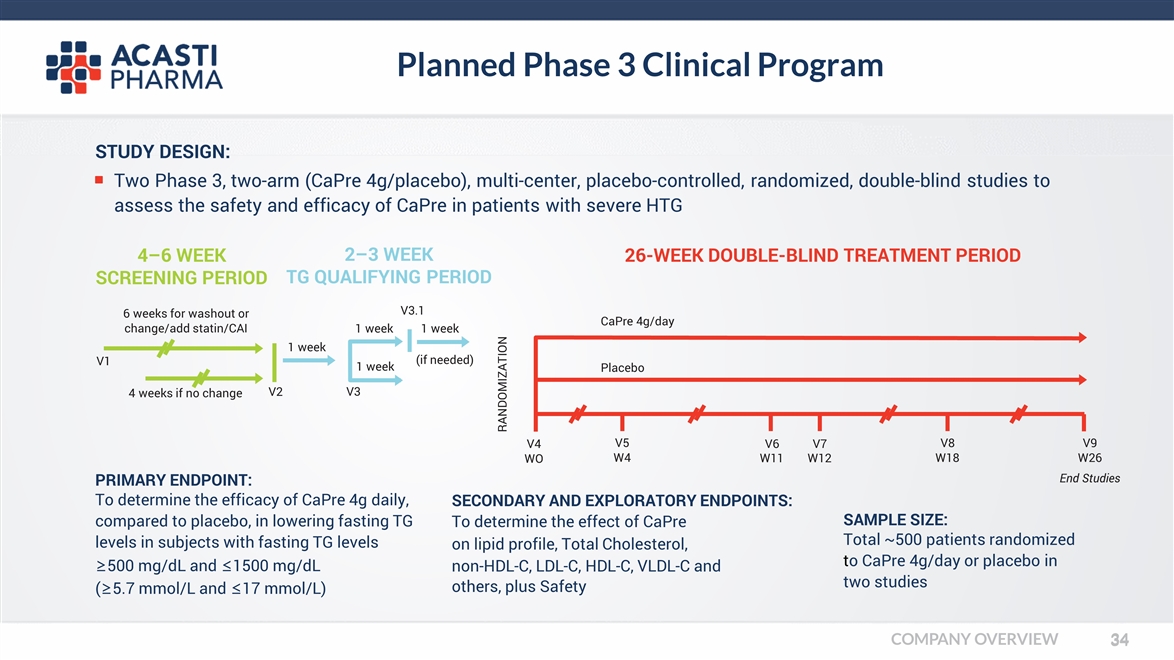
PRIMARY ENDPOINT: To determine the efficacy of CaPre 4g daily, compared to placebo, in lowering fasting TG levels in subjects with fasting TG levels ≥500 mg/dL and ≤1500 mg/dL (≥5.7 mmol/L and ≤17 mmol/L) STUDY DESIGN: 4–6 WEEK SCREENING PERIOD V1 V2 V3 4 weeks if no change 1 week 2–3 WEEK TG QUALIFYING PERIOD 1 week (if needed) RANDOMIZATION 26-WEEK DOUBLE-BLIND TREATMENT PERIOD Placebo V4 WO V5 W4 V6V7 W11W12 V8 W18 V9 W26 End Studies SAMPLE SIZE: Total ~500 patients randomized to CaPre 4g/day or placebo in two studies Two Phase 3, two-arm (CaPre 4g/placebo), multi-center, placebo-controlled, randomized, double-blind studies to assess the safety and efficacy of CaPre in patients with severe HTG 6 weeks for washout or change/add statin/CAI 1 week 1 week V3.1 CaPre 4g/day SECONDARY AND EXPLORATORY ENDPOINTS: To determine the effect of CaPre on lipid profile, Total Cholesterol, non-HDL-C, LDL-C, HDL-C, VLDL-C and others, plus Safety Planned Phase 3 Clinical Program COMPANY OVERVIEW
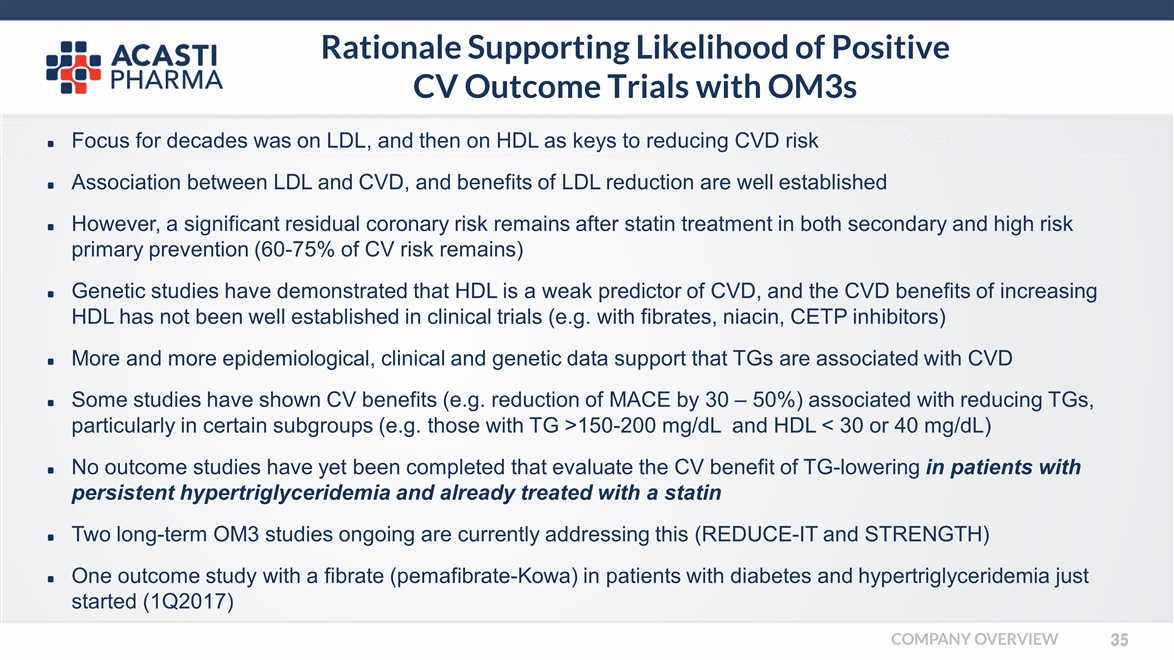
Rationale Supporting Likelihood of Positive CV Outcome Trials with OM3s Focus for decades was on LDL, and then on HDL as keys to reducing CVD risk Association between LDL and CVD, and benefits of LDL reduction are well established However, a significant residual coronary risk remains after statin treatment in both secondary and high risk primary prevention (60-75% of CV risk remains) Genetic studies have demonstrated that HDL is a weak predictor of CVD, and the CVD benefits of increasing HDL has not been well established in clinical trials (e.g. with fibrates, niacin, CETP inhibitors) More and more epidemiological, clinical and genetic data support that TGs are associated with CVD Some studies have shown CV benefits (e.g. reduction of MACE by 30 – 50%) associated with reducing TGs, particularly in certain subgroups (e.g. those with TG >150-200 mg/dL and HDL < 30 or 40 mg/dL) No outcome studies have yet been completed that evaluate the CV benefit of TG-lowering in patients with persistent hypertriglyceridemia and already treated with a statin Two long-term OM3 studies ongoing are currently addressing this (REDUCE-IT and STRENGTH) One outcome study with a fibrate (pemafibrate-Kowa) in patients with diabetes and hypertriglyceridemia just started (1Q2017) COMPANY OVERVIEW
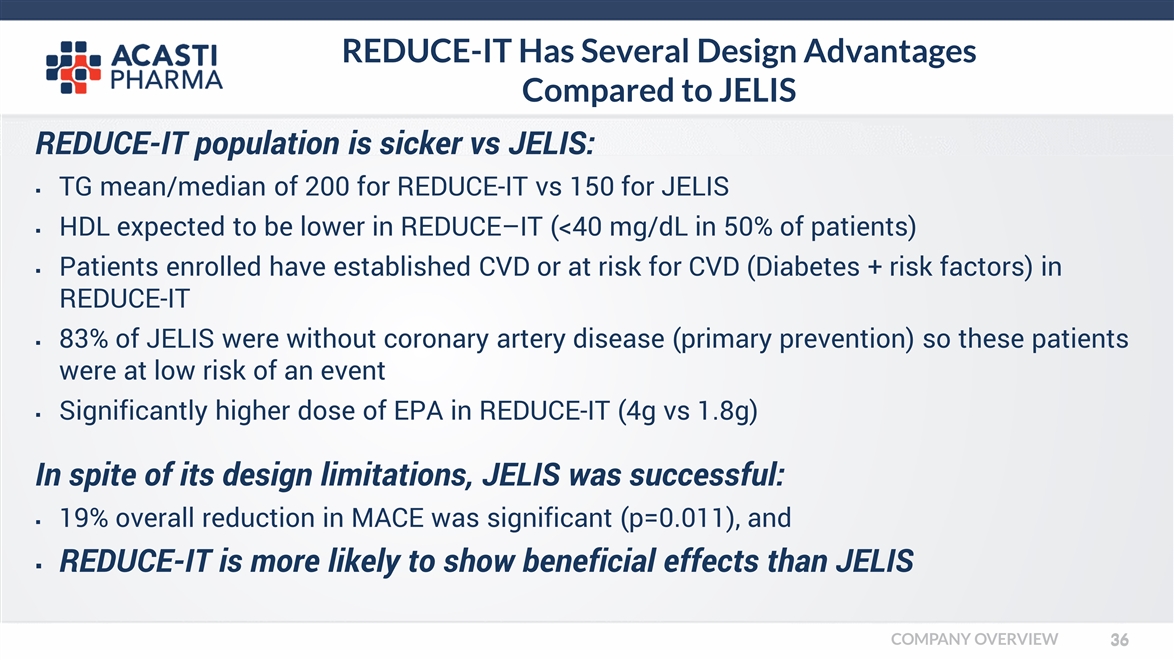
REDUCE-IT Has Several Design Advantages Compared to JELIS REDUCE-IT population is sicker vs JELIS: TG mean/median of 200 for REDUCE-IT vs 150 for JELIS HDL expected to be lower in REDUCE–IT (<40 mg/dL in 50% of patients) Patients enrolled have established CVD or at risk for CVD (Diabetes + risk factors) in REDUCE-IT 83% of JELIS were without coronary artery disease (primary prevention) so these patients were at low risk of an event Significantly higher dose of EPA in REDUCE-IT (4g vs 1.8g) In spite of its design limitations, JELIS was successful: 19% overall reduction in MACE was significant (p=0.011), and REDUCE-IT is more likely to show beneficial effects than JELIS COMPANY OVERVIEW
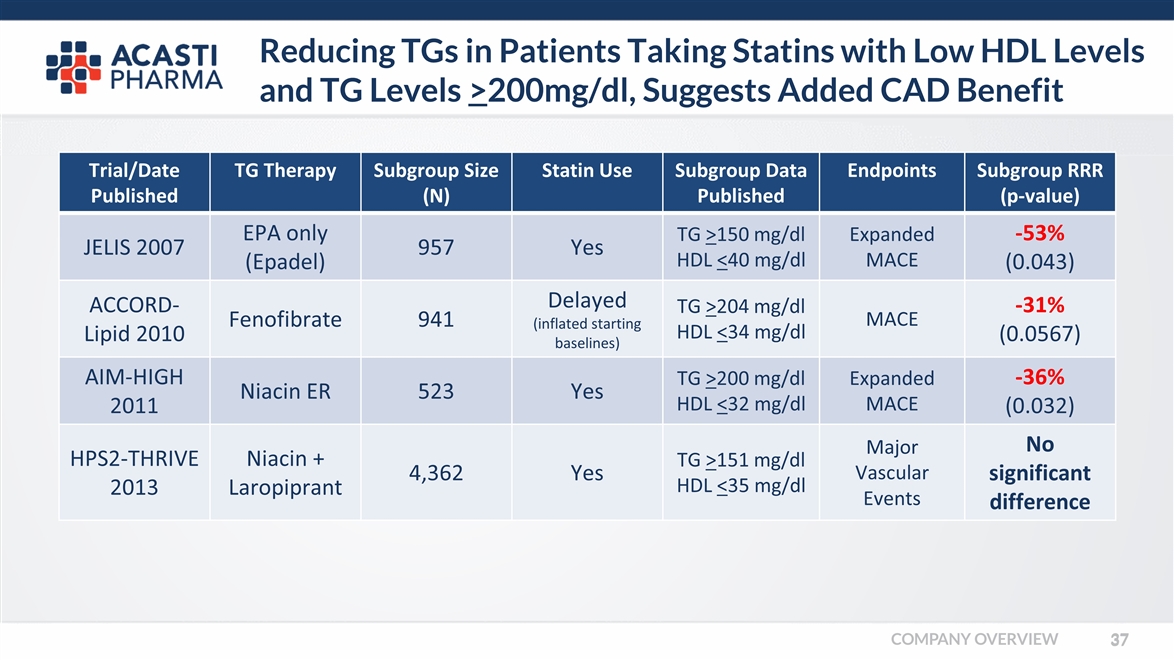
Reducing TGs in Patients Taking Statins with Low HDL Levels and TG Levels >200mg/dl, Suggests Added CAD Benefit Trial/Date Published TG Therapy Subgroup Size (N) Statin Use Subgroup Data Published Endpoints Subgroup RRR (p-value) JELIS 2007 EPA only (Epadel) 957 Yes TG >150 mg/dl HDL <40 mg/dl Expanded MACE -53% (0.043) ACCORD-Lipid 2010 Fenofibrate 941 Delayed (inflated starting baselines) TG >204 mg/dl HDL <34 mg/dl MACE -31% (0.0567) AIM-HIGH 2011 Niacin ER 523 Yes TG >200 mg/dl HDL <32 mg/dl Expanded MACE -36% (0.032) HPS2-THRIVE 2013 Niacin + Laropiprant 4,362 Yes TG >151 mg/dl HDL <35 mg/dl Major Vascular Events No significant difference COMPANY OVERVIEW

Positive Outcome Trials Could Be Major Catalyst for Significant OM3 Market Expansion If results of one or both of these two independent studies are positive, they could be significant value drivers for Acasti, especially if CaPre Phase 3 program confirms “best-in-class” effect on all major blood lipids Results of the following studies are highly anticipated for treatment of HTG with OM3 therapeutics. If successful, this could lead to improved medical care for tens of millions of patients and could significantly expand the potential global market opportunity REDUCE-IT: An on-going outcome study sponsored by Amarin is evaluating whether treatment with VASCEPA taken on top of a statin, reduces cardiovascular events in ~8,000 patients who have elevated TG levels and other cardiovascular risk factors. Over 400 clinical sites in 11 countries with the largest number of sites located within the United States. Results are expected mid-2018. STRENGTH: An on-going outcome study sponsored by AstraZeneca is evaluating whether EPANOVA taken on top of a statin, reduces cardiovascular events in ~13,000 patients with HTG. Results are expected in 2019. COMPANY OVERVIEW

Appendices COMPANY OVERVIEW

Scientific Advisors and Key Clinical/Regulatory Collaborators Robert Hegele, MD, FRCPC, FACP, FAHA, FCAHS Professor at Western University Director of London Regional Genomics Centre at Robarts Research Institute in London. Ont. Canada Robert H. Eckel, MD Charles A. Boettcher II Endowed Chair in Atherosclerosis Professor of Medicine - Division of Endocrinology, Metabolism and Diabetes, and Cardiology Director of Lipid Clinic, University of Colorado Hospital Barry A. Franklin, PhD, FAACVPR, FACSM, FAHA Director of Preventive Cardiology and Cardiac Rehabilitation Program at William Beaumont Hospital, Michigan Dariush Mozaffarian, MD, Dr. PH Dean Friedman School of Nutrition Science and Policy, Tufts University, Jean Mayer Chair and Professor of Nutrition Alexander G. Fleming, MD President and Chief Executive Officer of Kinexum COMPANY OVERVIEW
Industry 101: Breakdown of the Science What is hypertriglyceridemia (HTG)? Hypertriglyceridemia is a condition in which there are excess or elevated triglyceride (TG) levels in the blood. In layman's terms this means that fat levels in the blood are elevated to dangerous levels, and even with diet and lifestyle changes, they may not be reduced to a safe level. High levels of TGs can lead to diabetes, coronary heart disease, renal disease, and pancreatitis which is life-threatening. What are TGs and why are they bad? TGs are a type of fat found in your blood which your body uses for energy. Normal levels (in mg/dL) are < 150, border line = 150-199, high > 200 – 499 and very high is > 500. High levels can be caused by obesity, poorly controlled diabetes, an underactive thyroid, eating more calories than you burn, excess consumption of alcohol, certain medications and genetics. One can reduce one’s TGs by eating a healthy diet with limitations of fats and sugars, weight loss, quitting smoking, and more exercise. What is LDL-C, HDL-C, and non HDL-C? Low density lipoprotein (LDL-C) is the “bad cholesterol” in our bodies. LDL-C is an important measure for assessing the risk of heart disease, and can be controlled by diet and medication. High density lipoprotein (HDL-C) is the “good cholesterol” in our blood, which helps to remove LDL-C from our blood vessels. High levels of HDL-C have been associated with lower risk of heart disease, however more recent studies have shown that this correlation if it exists at all, is actually very weak. Non HDL-C is the measure of one's total cholesterol minus the HDL-C, which is an indication of the “bad” fats that are circulating in one's blood. High Non HDL-C is correlated with higher risk of coronary artery disease. COMPANY OVERVIEW

An Industry 101 Breakdown Continued… What role do OM3 fatty acids play? Clinical studies conducted with OM3 drugs showed their safety and efficacy in patients with HTG. For now, the FDA has approved OM3 drugs for the treatment of severe HTG. Outcome studies are currently ongoing to assess the safety and efficacy of OM3 drugs taken with a statin in patients with mild to moderate HTG. If these outcomes studies are positive, the market could be significantly expanded to the ~36 million people in the US with mild to moderate HTG, although FDA approval would first be required. Difference between OTC OM3s vs prescribed OM3s: Many types of OM3 non-prescription dietary supplements are available; however, the efficacy, quality, and safety of these products are open to question because they are not regulated by the same standards as pharmaceutical agents. These dietary supplements often contain high levels of triglycerides, which is the very substance that these patients are trying to reduce in their blood. Prescription OM3s can be used with confidence by practitioners who want to provide therapeutic doses of OM3s in a preparation that has been documented to be both safe and effective. Krill oil vs fish oil OM3s: Krill oil is a superior source of EPA and DHA, the two major OM3s, because these polyunsaturated fats are packaged as phospholipids, which can be used immediately by your body and is very efficiently absorbed. In addition, CaPre also contains EPA and DHA as free fatty acids that are rapidly absorbed. The EPA and DHA in fish oil dietary supplements, are typically packaged as TGs and must undergo additional chemical processing in order to remove the TGs for therapeutic use. This chemical process is called “esterification”, and results in an OM3 product that requires significant amounts of digestive enzymes (typically from a high fat meal) in order to fully release the OM3s into the intestine and be absorbed in the bloodstream. If a patient maintains a low-fat diet as should be the case in HTG, the OM3s in these Rx OM3 fish oils are less bioavailable, and therefore absorption is less efficient. COMPANY OVERVIEW

SCHEDULE A – Market Research Methodology and Assumptions DP Analytics, A Division of Destum Partners (“Destum”), an independent market research firm, conducted a qualitative U.S. commercial and market assessment for Acasti dated August 19, 2016, which is referred to in this presentation as the “Primary market research with Key Opinion Leaders (KOLs), High Volume Prescribers (HVPs) and Pharmacy Benefit Managers (PBMs)” Destum utilized secondary market research and primary qualitative market research with physicians and third party payer PBMs to conduct the analysis for CaPre: 1-on-1 in-depth phone interviews were conducted with 22 physicians and 5 PBMs Key data was obtained on current clinical landscape, unmet medical need, and commercial potential of CaPre Destum utilized OM3 prescription data from 2009-2015 for forecasting purposes COMPANY OVERVIEW

SCHEDULE A – Market Research Methodology and Assumptions, Continued Based on the PBM interviews, Destum assumed CaPre would be viewed favorably by payers at launch (e.g. Tier 2 or 3 depending on payer plan, which is comparable to VASCEPA) Upon completion of the screening questionnaire and approval for inclusion in the study, KOLs/HVPs were provided: Product X Target Product Profiles (Physicians interviewed were asked to assume a target product profile offering a “trifecta” of cardiometabolic benefits, i.e. effective lowering of TGs and non HDL-C, a neutral effect on LDL-C with the potential to lower LDL, and the potential to increase HDL-C, and that the potential efficacy and safety benefits demonstrated by CaPre in two Phase 2 clinical trials are generated and confirmed with statistical significance in a Phase 3 study to assess the safety and efficacy of CaPre in patients with severe hypertriglyceridemia. Such a target product profile was used by Destum Partners strictly for market research analysis purposes and should not be construed as an indication of future performance and should not be read as any form of expectation or guarantee of future performance or results, and will not necessarily be an accurate indication of whether or not such results will be achieved by CaPre® in any Phase 3 study) Study Questionnaire The interviews were administered via one-on-one in-depth phone interviews lasting on average 30-60 minutes All interviews were conducted by the same individual at Destum Partners to ensure consistency and collection of key data points COMPANY OVERVIEW
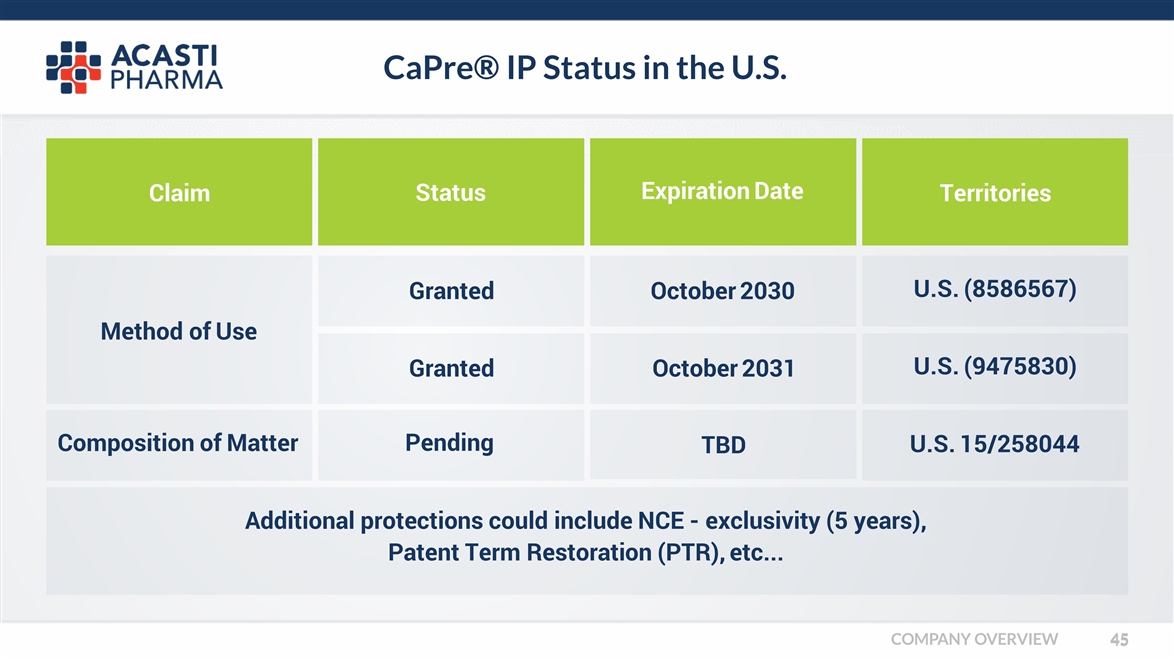
CaPre® IP Status in the U.S. Claim Status Expiration Date Territories Additional protections could include NCE - exclusivity (5 years), Patent Term Restoration (PTR), etc... Granted Granted Pending TBD U.S. 15/258044 October 2030 October 2031 U.S. (8586567) U.S. (9475830) Method of Use Composition of Matter COMPANY OVERVIEW
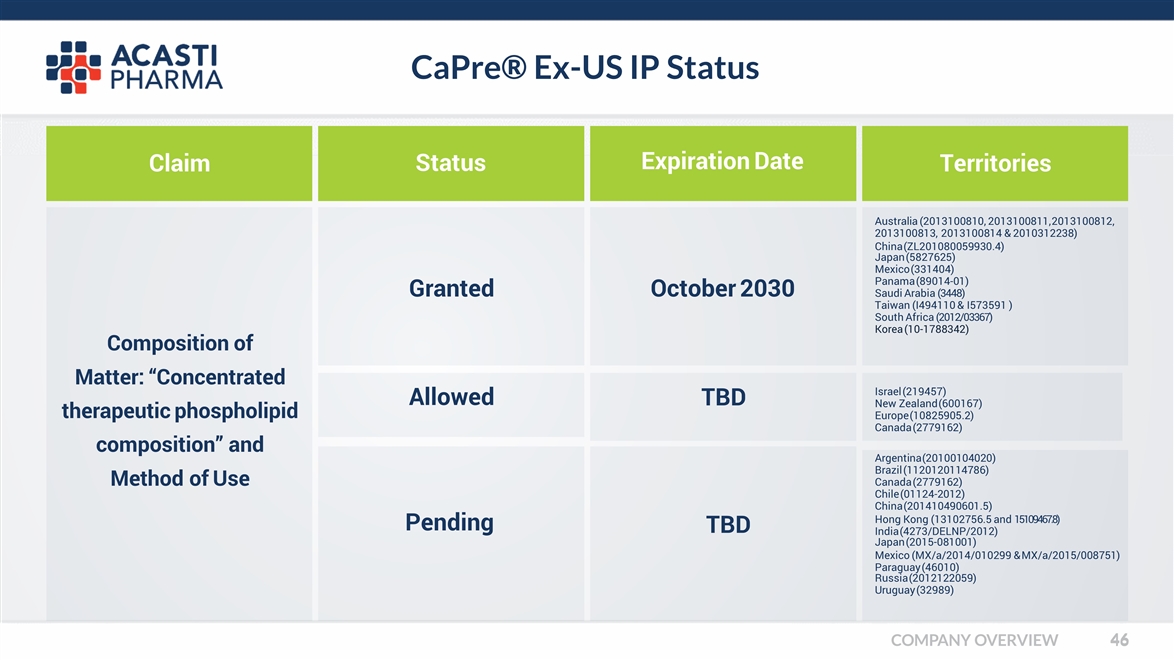
CaPre® Ex-US IP Status Claim COMPANY OVERVIEW Status Expiration Date Territories Granted Allowed Pending TBD TBD Argentina (20100104020) Brazil (1120120114786) Canada (2779162) Chile (01124-2012) China (201410490601.5) Hong Kong (13102756.5 and 15109467.8) India (4273/DELNP/2012) Japan (2015-081001) Mexico (MX/a/2014/010299 & MX/a/2015/008751) Paraguay (46010) Russia (2012122059) Uruguay (32989) Australia (2013100810, 2013100811, 2013100812, 2013100813, 2013100814 & 2010312238) China (ZL201080059930.4) Japan (5827625) Mexico (331404) Panama (89014-01) Saudi Arabia (3448) Taiwan (I494110 & I573591 ) South Africa (2012/03367) Korea (10-1788342) October 2030 Composition of Matter: “Concentrated therapeutic phospholipid composition” and Method of Use Israel (219457) New Zealand (600167) Europe (10825905.2) Canada (2779162)